Inadequate Protein Intake after Bariatric Surgery: Effects on Body Composition and Sarcopenic Obesity
 This activity has expired.
This activity has expired.
Course Overview: Despite the American Society for Metabolic and Bariatric Surgery (ASMBS) Allied Health Nutritional Guidelines for the Surgical Weight Loss Patient published in 2008 (Aills L, Blankenship J, Buffington C, Furtado M, Parrott J. ASMBS Allied Health Nutritional Guidelines for the Surgical Weight Loss Patient. Surg Obes Relat Dis. 2008;4(5 Suppl):S73–108.), current research has shown that as much as 64 percent of postoperative bariatric and metabolic surgical patients are not reaching the recommended daily protein intake. This article addresses the physiological need and assessment of clinical deficiencies as well as the recommended quality and intake following surgery.
Course Description: This continuing education course is designed to educate, through independent study, integrated healthcare clinicians who care for the metabolic and bariatric surgical patient population.
Course Objectives:
Upon completion of this program, the participant should be able to:
- Define sarcopenic obesity
- List the clinical signs and symptoms of inadequate protein intake following bariatric and metabolic surgery
- Explain the influencing factors that impact protein digestion and absorbability postoperatively.
- List the clinical signs and symptoms of inadequate protein intake following bariatric and metabolic surgery
- Define protein digestibility corrected amino acid score (PDCAAS)
- Discuss interventions to improve patient adherence for recommended protein quality and amount following bariatric and metabolic surgery.
Target Audience: This accredited program is intended for nurses and dietitians who treat patients with overweight or obesity.
Completion Time: This educational activity is accredited for a total of 1.0 contact hour (Nursing) and 1.0 CPEU (Dietetics)
Funding: This continuing education activity was supported by Bariatric Advantage (Aliso Viejo, California).
Disclosures: Cassie I. Story, RDN, is a clinical science liaison for Bariatric Advantage (Aliso Viejo, California) and a scientific advisor for Apollo EndoSurgery (Austin, Texas).
Provider: This educational program is provided by Matrix Medical Communications. Provider approved by the California Board of Registered Nursing, Provider Number 14887, for 1.0 contact hour and the Commission on Dietetic Registration, the credentialing agency for Academy of Nutrition and Dietetics, (Continuing Professional Education Accredited Provider Code E4B01A2) for 1.0 CPEU.
Provider Contact Information: Amanda Tolvaisa, Matrix Medical Communications, 1595 Paoli Pike, Suite 201, West Chester, PA 19380; Email: [email protected]
A Message from the Department Editor
Dear Colleagues:
I am very excited to introduce a four-part series on nutrition for the bariatric and metabolic surgical patient. Bariatric and metabolic surgery continues to be the most effective treatment for severe obesity, metabolic syndrome, and a host of life-threatening comorbidities. However, despite multiple clinical benefits, nutritional complications can develop in both the short-and long-term postoperatively if the patient does not take adequate supplementations. The vast majority of these potential deficiencies can be avoided with thorough preoperative and postoperative assessment, patient education, and diligent follow up. This article highlights the importance of guiding the patient to consume adequate, high-quality protein supplementation to support optimal outcomes that will benefit the patient both short and long term. The first in this series will discuss the role of protein. The series will continue with a focus on bone health, anemia treatment and prevention, and neurological health.
I hope you enjoy this detailed article on protein and make any necessary changes in your practice to ensure the best outcomes for your patients.
My Best to You,
Tracy Martinez RN, BSN, CBN
Bariatric Times. 2018;15(11):12–16.
By Cassie I. Story, RDN
Ms. Story is Private Practice in Scottsdale, Arizona
Funding: This continuing education activity was supported by Bariatric Advantage (Aliso Viejo, California).
Disclosures: Cassie I. Story, RDN, is a clinical science liaison for Bariatric Advantage (Aliso Viejo, California) and a scientific advisor for Apollo EndoSurgery (Austin, Texas).
Abstract: There is an urgent need for science-based nutrition recommendations following bariatric surgery. While evidence indicates that bariatric surgery is the most effective treatment option for the patient population with overweight or obesity, the increase in reported deficiencies and complications due to inadequate nutrition status postoperatively is cause for alarm. This four-part series will dive deeper into the following core areas of concern: 1) sarcopenic obesity and unfavorable body composition changes, 2) mineral and bone disorders, 3) anemias, and 4) neurologic complications. Though there are other numerous harmful bodily conditions that nutrient deficiencies can cause, or exacerbate, this series will focus on those that are most prevalent to this patient population.
The first article in this series will focus on sarcopenic obesity and unfavorable body composition changes by reviewing current evidence on the impact of dietary protein as well as review literature surrounding typical protein intake, and assess patient risk if they do not meet the minimum daily protein goal.
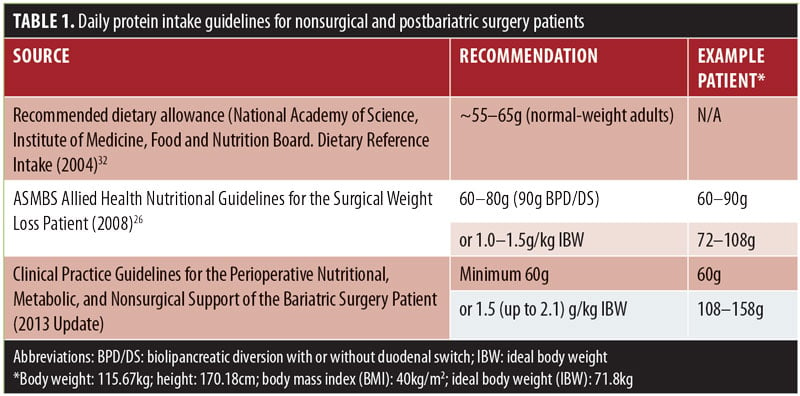
While there is extreme variance in postsurgical nutrition recommendations across the world, one nutrition recommendation that is routinely prescribed by the majority of healthcare providers who treat postoperative bariatric surgery patients is to consume a high-protein diet. Current guidelines for protein in the postbariatric patient suggest a range of 60 to 120g per day by encouraging protein with meals and protein supplementation,1 with individualized protein goals guided by a registered dietitian (RD) being considered best practice (Table 1).2
Proteins serve as the major structural component of muscle and other tissues in the body. In general, adequate protein is needed to preserve lean muscle mass (and facilitate the recovery of muscle function), improve or maintain nutritional status, increase satiety and feelings of fullness, support immunity and maintain weight loss.3,4 These functions are especially important to the postbariatric surgery patient, as their reduced food intake coupled with increased protein needs makes them more vulnerable than the general population for negative consequences of inadequate intake.
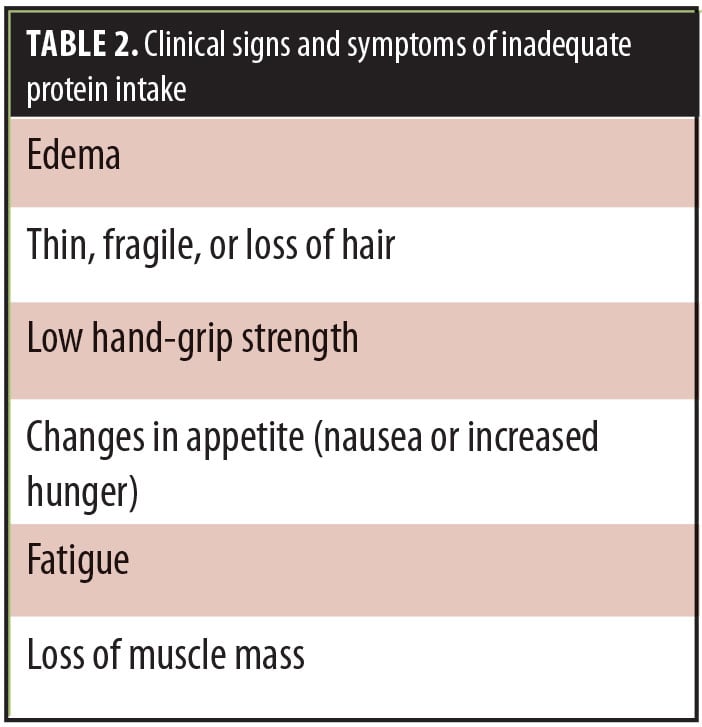
Clinical signs and symptoms of inadequate protein intake include the following (Table 2):
- Edema
- Thin, fragile, or loss of hair
- Low hand-grip strength
- Changes in appetite (nausea or increased hunger)
- Fatigue
- Loss of muscle mass
Reported Protein Intake Postbariatric Surgery
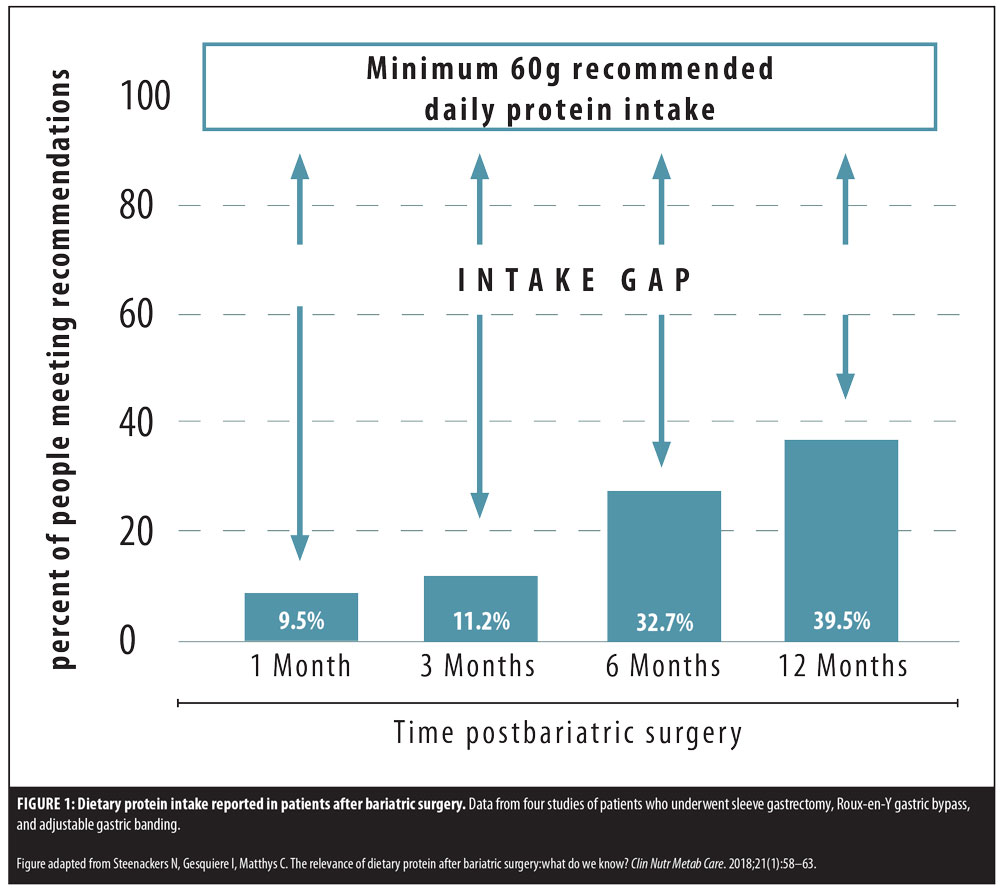
Immediately after bariatric surgery, an extreme decrease in food intake is expected due to reduced stomach capacity, changes in gut hormone secretion,5 and potential food intolerances and aversion.6 Despite a somewhat universal recommendation by healthcare providers who treat bariatric surgery patients to recommend consumption of a high-protein diet, studies indicate that postoperative patients often fall well below the minimum daily protein goal, and that the majority are not able to consume 60g of protein per day in both the short and long term7 from food sources alone.8
In a patient sample of 101 consecutive patients undergoing Roux-en-Y gastric bypass (RYGB) or sleeve gastrectomy (SG),9 37 to 61 percent of patients were unable to consume the minimum protein goal 12 months following bariatric surgery. A recent systematic review10 included protein intake data from patients who underwent RYGB, SG, or adjustable gastric band (AGB). The authors found that, at 12 months after surgery, between 46 and 64 percent of patients were not meeting the minimum protein goal of 60g per day, and reviewed one paper with 36-month data that showed 57 percent of RYGB patients were still not meeting minimum protein goals (Figure 1). The current research on protein intake following bariatric surgery clearly shows that while practitioners likely assume that protein intake increases to prescribed goals as the patient progresses from surgery, due to an increase in food volume capacity, the majority of patients do not ever reach the minimum daily protein goal, putting them at risk for long-term complications.
Not only is the minimum protein intake not achieved by most patients, there are unique circumstances that potentially impact digestion and absorption of dietary protein following bariatric surgery. Due to anatomical changes following the majority of procedures performed today, specifically the reduction in stomach capacity, hydrochloric acid, digestive enzymes, and potential rearrangement of the small intestine, protein digestion and absorption may be impacted.11
The effect of protein intake and its subsequent role in protein status remains difficult to evaluate. Protein deficiency after bariatric surgery can be influenced by several factors, including insufficient protein intake10; however, serum proteins are considered to be of limited value to assess nutritional status postoperatively,12 making identification of a mild-to-moderate protein deficiency difficult for the practitioner to diagnose. Outside of serum markers, body composition changes can be assessed to ensure adequate protein intake; however, limitations in clinical ease exist due to potential inaccuracies of in-office electric bioelectrical impedance analysis (BIA) scales.13
Clinical Considerations
Unfavorable body composition changes after bariatric surgery. Goals of bariatric surgery include improved health outcomes and body weight loss. All weight loss interventions result in losses of both fat mass (FM) and fat free mass (FFM); with a goal of the majority of body weight loss coming from adipose tissue while sparing lean muscle mass. Indeed literature surrounding FFM loss after bariatric surgery show a variance in reported rates between 11 and 31 percent.14,15 In clinical practice, a loss of 25 percent or less of FFM seems to be a reasonable goal; however, there is no “gold standard” and the range of acceptable FFM loss postbaritric surgery unknown.16
Changes in body composition after bariatric surgery have been an area of interest for many researchers, with higher protein intake17 as well as physical activity16 found to favorably impact FFM. The ability to preserve muscle function and mass postoperatively is of high priority in postbariatric surgery patients. Preservation of lean mass is critical since nonadipose tissues play a key role in resting metabolic rate and weight maintenance,18 and strategies to minimize the loss of lean body mass should be discussed with patients for successful weight management after surgery.
Certain weight loss interventions, including RYGB and biliopancreatic diversion with or without duodenal switch (BPD/DS) have been found to produce larger losses of FFM in the immediate postoperative period10 than nonsurgical weight loss interventions, likely due in part to the rapid weight loss that occurs after these procedures. However, evidence suggests that high protein intake protects against greater than expected loss of lean mass after surgery.19
A recent publication evaluating protein intake and its impact on FFM 12 months after SG20 found that the majority of patients did not reach a daily protein intake of 60g at each time point studied (6 and 12 months). They concluded that a protein intake greater than 60g per day was associated with lower relative FFM loss compared to a protein intake less than 60g and that inadequate protein intake following SG was associated with greater FFM loss.
Sarcopenic obesity. Consuming adequate protein intake helps protect against muscle catabolism (i.e., breaking down of muscle tissue) as seen in sarcopenic obesity, a relatively new medical condition characterized by the convergence of the aging population and the obesity epidemic. Individuals with sarcopenic obesity experience both a decrease in skeletal muscle mass and function (sarcopenia) and an increase in fat mass (obesity). Low muscle mass and quality (i.e., muscle strength relative to muscle size) have been found to have a negative impact on people with obesity and may lead to frailty, disability, and increased morbidity and mortality.21
Diagnosis. Current diagnostic tools for sarcopenia are limited in accuracy. Dual-energy X-ray absorptiometry (DXA) scans are one of the most accurate tools used for measuring bone density, but the technology is often not readily available and DXA scans are only recommended every two years postbariatric surgery.1
In-office measurement of body composition using BIA scales are considered acceptable in terms of accuracy if the patient is weighed following certain conditions as listed by the manufacturer (e.g., 12 hours fasting, adequate hydration status, no recent strenuous activity). It is recommended to maintain the same testing conditions from test to test for a true comparison of body composition changes.
For this reason, it is important to note time of day when using BIA in the office if comparisons are going to be made between appointments. For example, if looking for body composition change trends in individual patients, there will be great variance in results if the patient is seen at 8am one month and 4pm the following month, resulting in incomparable assessment. Additional types of tests include functional measures, such as hand-grip or walking tests, which can be assessed with qualified medical staff in the office.
Prevention and treatment. Multimodal approaches to preventing sarcopenic obesity and unfavorable body composition changes after surgery should include nutrition, physical activity, and medical monitoring. A large body of evidence exists to provide strong support of the association between protein intake with muscle growth as well as maintenance of lean body mass.21 The current bariatric surgery protein recommendations of 1.0 to 1.2g/kg of ideal body weight (IBW) seem reasonable and safe, with higher amounts needed for high-risk patients, including pregnant women and individuals with end-stage renal disease. Additionally, physical activity has been repeatedly shown to improve muscle function and mass, and individualized exercise recommendations should be provided to patients, ideally by an exercise physiologist within the bariatric practice.
Protein calorie malnutrition. Serum total protein and albumin levels are routinely monitored after bariatric surgery as a way to assess nutritional status, however several factors influence albumin levels (e.g., hydration, inflammation). The prevalence of hypoalbuminemia (<3.5g/dL) is relatively low after RYGB, particularly when limb length does not exceed 150cm,31 although one study26 reported rates of deficiency for distal gastric bypass as high as 13 percent.26
Bariatric surgery alone does not typically cause protein deficiency. Protein energy malnutrition (PEM) is usually associated with other conditions or circumstances including the following: anorexia, prolonged vomiting, diarrhea, food intolerance, depression, fear of weight regain, alcohol/drug abuse, and socioeconomic status.26 Although extreme cases of PEM are present and reported, PEM is not a common nutrition diagnosis after RYGB or SG.
Supplemental Protein
Mounting evidence suggests that patients do not reach protein intake recommendations with diet alone, and that more often than not, quality protein supplementation is needed on a daily basis to fill this gap and provide adequate nutrition.
In a systematic review published in 2017 evaluating the effect of protein intake on protein status and lean mass of postbariatric patients,12 the authors found that of 12 studies reviewed (RYGB [n=539]; SG [n=142]; range of time postoperative= 1–36 months), the only patients who were able to reach at least 60g of protein intake per day used protein supplements plus a high-protein diet. Nine of the 12 studies did not recommend protein supplements and found that patients were unable to reach a goal of 60g of protein per day through food sources.
Some clinicians might hesitate to recommend protein supplements to patients, citing various reasons, such as a patient’s preference to receive protein from food sources alone instead of relying on supplements.
The evidence clearly shows that, for the majority of patients, intake through dietary sources of protein alone results in less than 60g of protein per day. Typically, low protein intake puts postbariatric patients at unnecessary risk for unfavorable body composition changes, weight regain, sarcopenic obesity, low immune function, and, in severe cases, protein calorie malnutrition.
Consuming quality protein supplements can help patients meet daily protein goals without significantly increasing the consumption of additional calories. Proteins are made up of amino acids. There are 21 known amino acids, nine of which cannot be made by the body and are classified as essential. A group of essential amino acids known as branched-chain amino acids (BCAA) are known to be important for muscle protein synthesis and to prevent muscle breakdown.22
The protein digestibility-corrected amino acid score (PDCAAS) can be used to determine the quality and digestibility of a protein.23 Scores range from 0.0 (low quality) to 1.0 (high quality). Examples of protein types and their PDCAAS are as follows:
- Hydrolyzed collagen protein: 0.0
- Rice protein: 0.47
- Pea protein: 0.73
- Soy protein: 1.0
- Whey protein (complete in BCAAs): 1.024
Whey protein is typically sold as concentrate or isolate. Isolates are the purest protein source available, containing almost no carbohydrates, lactose, or fats. Patients who are lactose intolerant can often safely use whey protein isolate.25
Special Considerations for Higher Risk Patient Populations
There are various subsets of the postbariatric surgery patient population, such as pregnant women and individuals with end-stage renal disease, that are at a higher or unique nutrition risk. Clinicians should be familiar with the unique needs of certain patients.
Pregnancy. It is estimated that up to 80 percent of bariatric procedures are performed on women of childbearing age.27 Several papers in recent years have highlighted that, although there are favorable conception outcomes for women postbariatric surgery, the maternal and neonatal outcomes due to nutrition deficiencies cause by these procedures carry some risk.
Evidence shows that a previous RYGB carries a risk of infants being small for gestational age (SGA) compared to matched controls.28 Studies on the impact of SG are beginning to emerge, and a recent report found a three-fold increased risk for SGA infants among 119 post SG pregnant patients compared to controls.27
A recent study has shown the same findings when comparing RYGB to SG and concluded that each procedure carried similar fetal growth restrictions.29 The authors of the study also noted that protein intake had a positive impact on fetal growth, while noting that the majority of study participants did not reach the minimum goal of 60g protein per day, with RYGB patients averaging 59g per day, and SG averaging 47g per day. The time to conception from surgery in this study was 31 months for RYGB and 24 months for SG, which is well within the current practice guidelines that suggest delaying conception to 12 to18 months.2 In the same study, 95 percent of RYGB patients were taking a multivitamin, while presenting with an average of seven nutritional deficiencies and that 80 percent of SG patients were taking a multivitamin, while presenting with an average of six nutritional deficiencies, thus highlighting the need for proper bariatric-specific supplement recommendations and proper screening for nutrition deficiencies for all postbariatric surgery patients, especially those at risk increased risk for deficiencies.
End-stage renal disease. Patients receiving dialysis have higher protein needs to mitigate the nutrition losses that exist with dialysis treatment and to prevent protein wasting and catabolism. For the postbariatric surgery patient receiving dialysis, special care should be given to their protein intake, with the knowledge of the protein intake gap that exists after surgery. Protein needs for someone receiving dialysis are 1.2g/kg dry weight, which may result in higher than recommended protein intake compared to traditional postbariatric surgery guidelines. Considerations for protein supplementation for the postbariatric surgery patient receiving dialysis are as follows:30
- 10 to 30g high biologic value protein per serving
- Less than 250mg potassium, 200mg phosphorus, and 300mg sodium
Conclusion
Quality of weight lost after bariatric surgery should be included in the postoperative assessment to evaluate body composition changes and nutritional status. Universalization of daily protein supplementation recommendations in the postbariatric surgery patient is needed. With universal guidelines in place, clinicians might better assist with patient adherence to minimum daily protein goals. Better patient adherence to protein recommendations would likely benefit patients by decreasing the risk of inadequate protein intake and promote prevention of unfavorable body composition changes, sarcopenic obesity, and unintentional weight regain.
References
- Mechanick JI, Youdim A, Jones DB, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient: Cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Obesity (Silver Spring). 2013;21 Suppl 1:S1–27.
- Mechanick J, Youdim A, Jones D, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient—2013 Update: Cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Surg Obes Relat Dis.2013;9:159–91.
- Diepvens K, Haberer D, Westerterp-Plantenga M. Different proteins and biopeptides differently affect satiety and anorexigenic/orexigenic hormones in healthy humans. Int J Obes (Lond).2008;32(3):510–518.
- Halton TL, Hu FB. The effects of high protein diets on thermogenesis, satiety and weight loss: a critical review. J Am Coll Nutr. 2004;23(5):373–385.
- Quercia I, Dutia R, Kotler DP, et al. Gastrointestinal changes after bariatric surgery. Diabetes Metab.2014;40(2):87–94.
- Zerrweck C, Zurita L, Alvarez G, et al. Taste and olfactory changes following laparoscopic gastric bypass and sleeve gastrectomy. Obes Surg. 2016;26:1296–1302.
- Moize V, Andreu A, Rodriguez L, et al. Protein intake and lean tissue mass retention following bariatric surgery. Clin Nutr. 2013;32(4):550–555.
- Beckman L and Earthman C. Nutritional implications of bariatric surgery and the role of registered dietitians. J Acad Nutr Diet. 2013;113(3): 398–399.
- Andreu A, Moize V, Rodriguez L, et al. Protein intake, body composition, and protein status following bariatric surgery. Obes Surg. 2010;20:1509–1515.
- Steenackers N, Gesquiere I, Matthys C. The relevance of dietary protein after bariatric surgery:what do we know? Clin Nutr Metab Care. 2018;21(1):58–63.
- Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2010;11:41–50.
- Ito M, Goncalves V, Faria S, et al. Effect of protein intake on protein status and lean mass of post-bariatric surgery patients: a systematic review. Obes Surg. 2017;27:502–512.
- Nicoletti C, Junqueira-Franco M, Ernesto dos Santos J, et al. Protein and amino acid status before and after bariatric surgery: a 12-month follow-up study. Surg Obes Relat Dis. 2013;9:1008–1012.
- Carey DG, Pliego GJ, Raymond RL. Body composition and metabolic changes following bariatric surgery: Effects on fat mass, lean mass and basal metabolic rate: six months to one-year follow-up. Obes Surg. 2006;16:1602–1608.
- Coupaye M, Bouillot JL, Coussieu C, et al. One-year changes in energy expenditure and serum leptin following adjustable gastric banding in obese women. Obes Surg. 2005;15:827–833.
- Chaston T, Dixon J, O’Brien P. Changes in fat-free mass during significant weight loss: a systematic review. Int J Obes. 2007;31:743–750.
- Westerterp-Plantenga M, Nieuwenhuizen A, Tome D, et al. Dietary protein, weight loss, and weight maintenance. Annu Rev Nut. 2009;29:21–41.
- Friedrich A, Dammus-Machado A, Meile T, et al. Laparoscopic sleeve gastrectomy compared to a multidisciplinary weight loss program for obesity-effects on body composition and protein status. Obes Surg. 2013;23(12):1957–1965.
- Schollenberger AE, Karschin J, Meile T, Kuper MA, Konigsrainer A, Bischoff SC. Impact of protein supplementation after bariatric surgery: A randomized controlled double-blind pilot study. Nutrition. 2016;32(2):186–192.
- Dagan S, Tovim T, Keidar A, et al. Inadequate protein intake after laparoscopic sleeve gastrectomy surgery is associated with a greater fat free mass loss. Surg Obes Relat Dis. 2018;13:101–110.
- Barazzoni R, Bischoff S, Boirie Y, et al. Sarcopenic obesity: Time to meet the challenge. Obes Facts. 2018;11:294–305.
- Volpi E, Kobayashi H, Sheffield-Moore M, et al. Essential amino acids are primarily responsisble for the amino acid stimulation of muscle prortein anabolism in healthy elderly adults. Am J Clin Nutr. 2003;78(2):250–258.
- Schaafsma G. The protein digestibility-corrected amino acid score. J Nutr. 2000;130(7):1865S–1867S.
- Phillips SM. Current concepts and unresolved questions in dietary protein requirements and supplements in adults. Front Nutr. 2017;4:13.
- Geiser M. The wonders of whey protein. NSCA’s Performance Training Journal. 2003;2(5):13–15.
- Aills L, Blankenship J, Buffington C, et al. ASMBS Allied Health Nutritional Guidelines for the Surgical Weight Loss Patient. Surg Obes Relat Dis. 4 (2008); S73–S108.
- Rottenstreich A, Elazary R, Levin G. Pregnancy after bariatric surgery and the risk of fetal growth restriction. Surg Obes Relat Dis. 2018 Sep 14. [Epub ahead of print]
- Kwong W, Tomlinson G, Feig DS. Maternal and neonatal outcomes after bariatric surgery; a systematic review and meta-analysis: do the benefits outweigh the risks? Am J Obstet Gynecol. 2018;218(6): 573–580.
- Coupaye M, Legardeur H, Sami O, et al. Impact of Roux-en-y gastric bypass and sleeve gastrectomy on fetal growth and relationship with maternal nutritional status. Surg Obes Relat Dis. 2018;000:1–7.
- Beto JA, Bansal VK. Medical nutrition therapy in chronic kidney failure: integrating clinical practice guidelines. J Am Diet Assoc. 2004;104(3):404–409.
- Ritz P, Becouarn g, Douay O, et al. Gastric bypass is not associated with protein malnutrition in morbidly obese patients. Obes Surg. 2009;19:840–844.
- National Academy of Science, Institute of Medicine, Food and Nutrition Board. Dietary Reference Intake. 2004. www.nal.usda.gov/fnic. Accessed October 3, 2018.
Category: Past Articles, Review







