Neurohormonal Effects on Obesity
by April Smith, PharmD, MA, BCPS
April Smith, PharmD, MA, BCPS, is Associate Professor of Pharmacy Practice at the School of Pharmacy & Health Professions at Creighton University in Omaha, Nebraska.
Funding: No funding was provided.
Disclosures: The author reports no conflicts of interest relevant to the content of this article.
Abstract: This article provides an overview of key peripheral peptides secreted from gut and adipose tissue that influence appetite and satiety. The author explores how and where these peptides interact with the hypothalamus, dorsal vagal complexes, and higher brain centers. By exploring this neurohormonal circuitry, readers should be able to identify target receptors that could influence treatment strategies for obesity. The role of neurohormonal peptides in weight regain and the impact of bariatric surgery on these peptides are also discussed.
Keywords: Ghrelin, glucagon-like peptide-1, peptide YY, leptin, insulin, amylin, arcuate nucleus, dorsal vagal complex, neurohormone, obesity
Bariatric Times. 2017;14(11):10–14.
Introduction
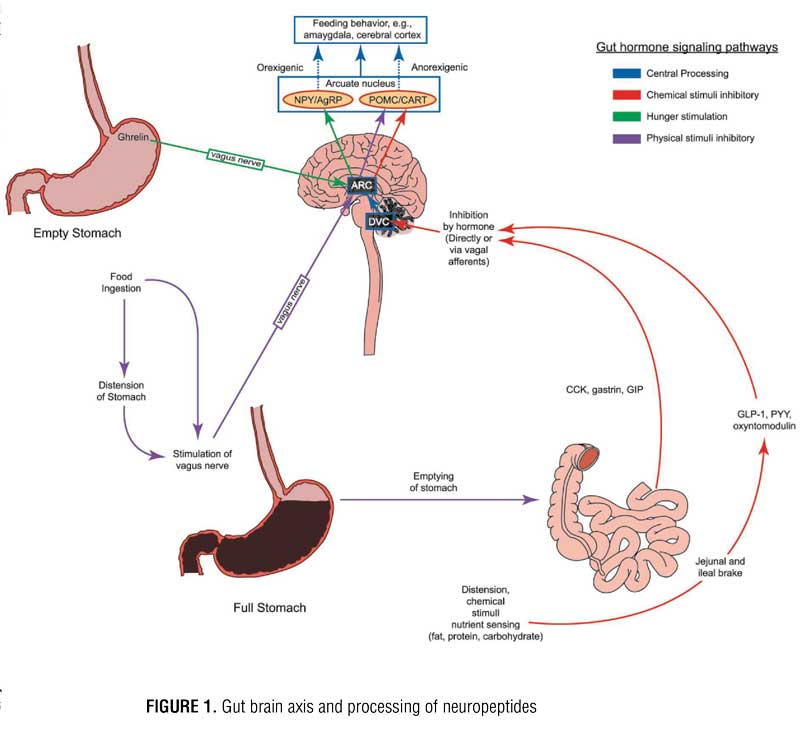
Regulation of appetite and food intake is mediated by the gut-brain axis, in which several gut hormones and neuropeptides travel, either directly through the blood brain barrier or indirectly via vagus nerve currents. This intricate circuitry between the gut and brain centers such as the dorsal vagal complex and the hypothalamus controls appetite, gastric motility, and satiety. Neuropeptide pathways originate from three systems/responses: 1) adipose tissue, 2) gut response to nutrient sensing from food, and 3) gut response to mechanical stretch from food. These pathways carry appetite-related signals to the brain.[1] Variations in levels of these neuropeptides between individuals with obesity and individuals with normal weight have been identified. This research has led to the development of drugs and devices that attempt to modulate various pathways in this interrelated neuropeptide signaling scheme in individuals with obesity. Bariatric surgery has been shown to have a variable, yet sometimes drastic and sustainable, impact on levels of key peptides, such as ghrelin, leptin, and glucagon-like peptide-1 (GLP-1).[2] To date, most research on the epigenetics of obesity has focused on peptide signaling that influences appetite and food intake rather than energy expenditure. The key appetite-regulating neuropeptides will be explored herein.
Hypothalamus and Brainstem Signal Processing to Control Food Intake
The hypothalamus is the major target for appetite regulating peripheral peptides released from adipose tissue and gastrointestinal tract organs. These peptides can travel through the blood stream, cross the blood brain barrier, and interact directly with the hypothalamus by stimulating the arcuate nucleus (ARC) or indirectly simulating the ARC upon delivery from vagus nerve afferents. During indirect communication, signals travel along the vagus nerve first to the dorsal vagal complex (DVC) in the brainstem, then to the ARC, paraventricular nucleus (PVN) of the hypothalamus, or to higher brain centers. Within the ARC, there are two groups of neurons: orexigenic (neuropeptide Y [NPY] and agouti-related peptide [AgRP] releasing neurons) and anorexigenic (pro-opiomelanocortin [POMC] and cocaine-amphetamine related transcript [CART] releasing neurons). Both groups of neurons within the ARC contain receptors for several different neuropeptides. When NPY/AgRP neurons are stimulated, they send signals to other regions of the hypothalamus, such as the PVN and the lateral hypothalamus, to induce appetite. When POMC/CART neurons are stimulated, they signal to the same secondary regions of the hypothalamus to suppress appetite. These messages are then translated to higher brain centers, such as the amygdala and cerebral cortex, and it is within this complex circuitry that eating behavior is ultimately driven (Figure 1).[3] Hedonic input is coordinated in higher brain centers, and a “reward” pathway is created with certain foods. Dopaminergic and NPY receptors appear to be most closely linked to eating for reward. It should be noted that food composition, particularly when high in sugar and fat, can trigger an altered response of these gut hormones, which might allow a strong connection with the reward pathway and promote continued feeding beyond the point of satiation;[4] however, further discussion of this is beyond the scope of this article.
Satiety Peptides and Response to Ingestion of Food
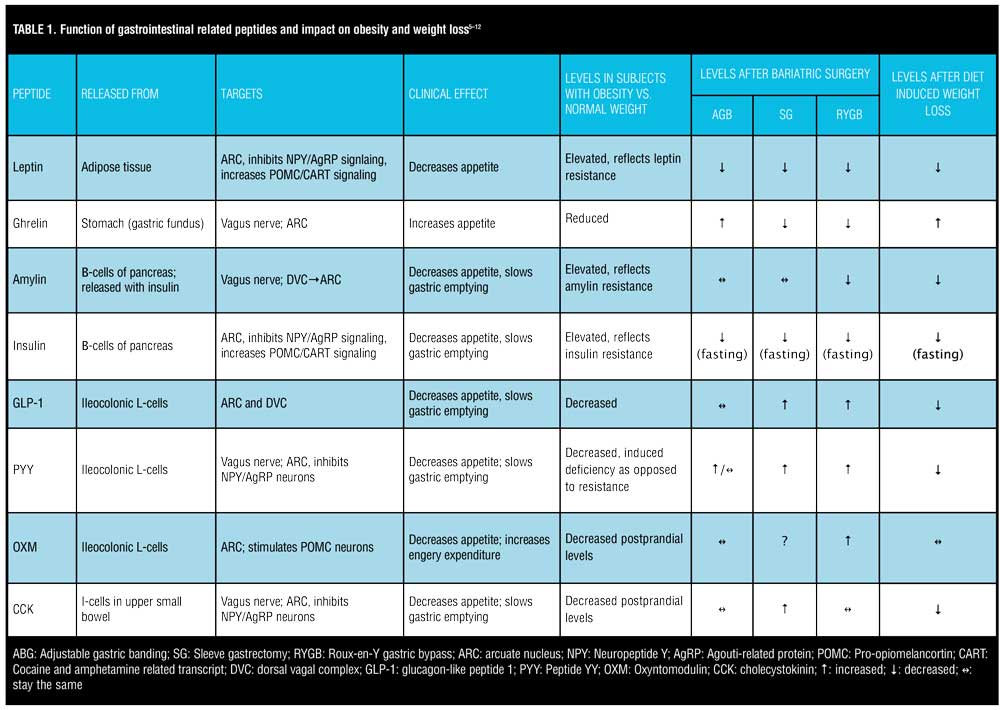
The ARC contains receptors for various peptides. Perhaps the most important receptor in terms of appetite regulation, however, are ghrelin, glucagon-like peptide 1 (GLP-1), peptide YY (PYY), oxyntomodulin (OXM), and leptin. Ghrelin is the body’s most well-known orexigenic hormone. Ghrelin peaks right before meals, decreases sharply postprandially, and accelerates gastric emptying. Ghrelin works in opposition of the anorexigenic/satiety peptides. Table 1 describes the various adipose and gastrointestinal secreted peptides, their sites of release, their target receptors, and their effects on appetite.
When food enters the stomach, the physical tension is sensed by vagal nerve afferents. Signals are then sent to the brain that cause the stomach to expand to accommodate the meal and contract to empty the meal through the pyloric sphincter. As food or chyme passes into the small bowel, enteroendocrine cells start to release GLP-1, cholecystokinin (CCK), and PYY in response to physical distension or sensing of nutrients. Once the level of nutrients reaches a certain point in the intestine, these peptides, mostly via negative feedback action in the brain, slow gastric emptying. The release of these peptides is also influenced by meal composition. The enteroendocrine “I” cells in the upper small bowel release CCK in response to food high in protein and fat. The enteroendrocrine “L” cells in the lower small bowel and colon release GLP-1 upon detection of carbohydrates and fats. GLP-1 is also an incretin that can stimulate the pancreas to secrete insulin in response to high carbohydrate intake and increased blood sugar levels.[7] CCK, PYY, and GLP-1 also induce satiety and, therefore, have an anorexigenic effect. While the incretin effect of hormones, such as insulin and amylin, are well known for the role they play in diabetes, their role in obesity to decrease food intake by stimulating POMC/CART neurons in the ARC is a more recent and intriguing discovery.[9] Oxyntomodulin (OXM) is released post-prandially along with GLP-1 from enteric L-cells and serves as an appetite suppressant but has little to no incretin effect like GLP-1. Exogenous administration of OXM has been shown not only to reduce food consumption but also increase energy expenditure.[6] Pre-prandial injections are a notable barrier to OXM’s utility as a weight loss agent. Blockade of the vagus nerve has been shown to interrupt signaling from these aforementioned peptides, which in turn prevents gastric distension, delays gastric emptying, and prevents communication of ghrelin with the hypothalamus to deliver its hunger message.
Adiposity and Hormones that Influence Energy Homeostasis
Adipose tissue produces various hormones, but the most influential in terms of appetite regulation and energy stores is leptin. Leptin was one of the first hormones that was recognized to highly influence the body’s fat storage. Mutations of the leptin gene result in morbid obesity, and increased expression of the gene results in energy expenditure and weight loss.[6] Leptin receptors are located in the ARC of the hypothalamus. Leptin inhibits NPY/AgRP neurons and stimulates POMC/CART neurons. Individuals with obesity display elevated levels of circulating leptin but without a corresponding clinical response of decreased appetite. This likely reflects leptin receptor resistance. Elevated, circulating levels of leptin and subsequent leptin resistance have been linked to pancreatic beta-cell dysfunction, insulin resistance, and interference with GLP-1 secretion.[13] Leptin could, therefore, play a significant role in the relationship between obesity and type 2 diabetes. Conversely, there is a drastic reduction in leptin levels following bariatric surgery, which leads to the resolution of type 2 diabetes. While GI peptides, such as GLP-1, PYY, CCK, and OXM, have more impact on short-term regulation of food consumption and are released in response to nutrient sensing in the gut, leptin has more of an impact on long-term energy intake.[14] Leptin appears to regulate energy intake, energy expenditure, and weight homeostasis.[15]
Fasting and postprandial levels of ghrelin are lower in patients with obesity versus those of normal weight.[7] “Grazing” type behavior in patients with obesity could in part be due to plateaued levels of ghrelin as opposed to the sharp drop in postprandial ghrelin in lean individuals. Postprandial levels of CCK, GLP-1, and PYY are reduced in patients with obesity versus lean subjects.[16] Considering that the primary role of these three gut peptides in appetite suppression is delayed gastric emptying, altered levels in patients with obesity could explain why they might feel less satiated after a meal.
Impact of Non-surgical versus Surgical-Induced Weight Loss on Appetite-Regulating Peptides
Several studies have examined the impact of diet and exercise —(non-surgical) induced weight loss on appetite regulating peptides as one method to explain why patients who achieve clinically significant weight loss (~4–10% of their body weight) with lifestyle modification alone regain roughly 30 percent of the weight lost within the first year.[6] Currently available evidence on weight loss-induced changes in appetite-regulating hormones supports a physiological-based rationale for why weight maintenance after a period of acute weight loss is so challenging. The disruption of gut hormones persists following acute weight loss, and levels of some key peptides never return back to pre-weight loss baseline even after one year.[11]
Given leptin’s release from adipose tissue, it is not surprising that reduction in body weight and fat mass result in decreased levels of leptin. Profound reduction in circulating leptin levels following acute diet-induced weight loss can cause an increase in appetite and a decrease in energy expenditure. Sumithran et al11 followed 50 patients with obesity through a 10-week diet-intensive weight loss program and then for one additional year. They found that after a sharp decrease in leptin levels during the 10-week diet phase, consistently decreased leptin levels were strongly correlated with weight regain over the one-year follow-up period.
Levels of anorexigenic gut hormones, such as GLP-1, PYY, and CCK, are all decreased from baseline following a period of diet-induced weight loss (Table 1). Reductions in these hormones can persist up to one year after diet-induced weight loss, which promotes increased appetite and decreased satiety, eventually leading to weight regain.[7] Higher fasting levels of ghrelin compared to baseline are observed after a period of diet-induced weight loss, increasing the desire to overeat. It appears the body tries to defend its original set point by increasing orexigenic peptides and decreasing anorexigenic peptides following a period of non-surgical-induced weight loss. This homeostatic imbalance might explain why there seems to be a limit to the amount of weight that can be lost via lifestyle modification alone and why maintaining the weight loss is so challenging. Medications and gene therapies directed toward moderating optimal levels of these peptides are currently available, and more are being investigated.
The role of gut-brain axis peptides in explaining the failure of diet alone to preserve weight loss compared to the persistent weight loss seen in bariatric surgery patients has been applicably explored. In contrast to diet alone, today’s most commonly performed bariatric surgery procedures (sleeve gastrectomy [SG] and Roux-en-Y gastric bypass [RYGB]) are associated with sustained reductions in orexigenic hormones and increases in anorexigenic hormones up to one year postoperatively.[17]
Leptin levels are reduced after all types of bariatric surgery, presumably because of the drop in body fat mass. Adipocyte reduction might not be the only mechanism of leptin reduction since a drop in leptin levels can be seen as soon as one week after SG and RYGB, prior to notable weight loss.[18] Leptin is also produced in the gastric fundus. Limited interaction of nutrients with the limited remaining gastric fundus after SG and RYGB could explain the early reduction in leptin levels.
Not all bariatric procedures have the same impact on these gut-derived appetite-regulating hormones. Much like diet-induced weight loss, adjustable gastric banding (AGB) was associated with increased ghrelin levels versus decreased levels seen after SG and RYGB. Favorable increases in anorexigenic hormones, such as GLP-1, PYY, and post-prandial insulin, are more pronounced in RYGB versus AGB subjects and seem to be similarly significant in RYGB and SG patients[8,12,19-20]. Compared to SG, RYGB patients exhibit higher post-prandial levels of GLP-1 and PYY, most likely because of earlier delivery of nutrients to the ileum[15]. Table 1 illustrates the advantageous impact of RYGB and SG on appetite-influencing gut peptides and how bariatric surgery enhances gut-brain circuitry to promote weight loss. This is in contrast to the adversarial neurohormonal environment created by diet-induced weight loss, which seems to put the body into a state of defending its original weight set point.
Conclusion
Physiological research on obesity has resulted in tremendous developments, and one of the most influential advances is the delineation of neurohormonal factors that interplay along the gut brain axis to control appetite, gastric emptying, satiety, and overall adiposity. Weight loss induced by diet alone is difficult to maintain, in part because of the increase in ghrelin and decrease in anorexigenic gut peptides, such as GLP-1, PYY, CCK, and amylin, produced by this method of weight loss. In contrast to diet alone or older purely restrictive procedures, today’s most popular bariatric surgery procedures (SG and RYGB), improve and enhance levels of anorexigenic gut peptides without increasing ghrelin. Future research on pharmacological, genetic, and surgical methods to better use these pathways in attempt to shift them toward a more anorexigenic promoting milieu will likely continue.
References
- Berthoud HR. The vagus nerve, food intake and obesity. Regul Pept. 2008; 149:15–25.
- Ochner CN, Gibson C, Carnell S, et al. The neurohormonal regulation of energy intake in relation to bariatric surgery for obesity. Physiol Behav. 2010;100(5):549–559.
- Camilleri M. Peripheral mechanisms in appetite regulation. Gastroenterology. 2015;148(6):1219–1233.
- Scalfani A. Gut-brain nutrient signaling: appetition vs. satiation. Appetite. 2013;71: doi:10.1016/j.appet.2012.05.024.
- Ochner CN, Gibson C, Shanik M, et al. Changes in neurohormonal gut peptides following bariatric surgery. Int J Obes (Lond). 2011;35(2):153–166.
- Kim GW, Lin JE, Valentino MA, et al. Regulation of appetite to treat obesity. Expert Rev Clin Pharmacol. 2011;4(2):243–259.
- Lean MEJ, Malkova D. Altered gut and adipose tissue hormones in overweight and obese individuals: cause or consequence? Int J Obes. 2016;40:622–632.
- Ramón JM, Salvans S, Crous X, et al. Effect of Roux-en-Y gastric bypass vs sleeve gastrectomy on glucose and gut hormones: a prospective randomised trial. J Gastrointest Surg. 2012; 16:1116–1122.
- Reda TK, Geliebter A, Pi-Sunyer X. Amylin, food intake, and obesity. Obes Res. 2002; 10(10):1087–1091.
- Laferrere B, Swerdlow N, Bawa B, et al. Rise of oxyntomodulin in response to oral glucose after gastric bypass surgery in patients with type 2 diabetes. J Clin Endocrinol Metab. 2010;95(8):4072–4076.
- Sumithran P, Prendergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Eng J Med. 2011;365:1597–604.
- Malin SK, Kashyap SR. Differences in weight loss and gut hormones: Rouxen-Y gastric bypass and sleeve gastrectomy surgery. Curr Obes Rep. 2015;4:279–286.
- Yong-ho L, Magkos F, Mantzoros CS, et al. Effects of leptin and adiponectin on pancreatic β-cell function. Metabolism. 2011;60:1664–1672.
- Owyang C, Heldsinger A. Vagal control of satiety and hormonal regulation of appetite. J Neurogastroenterol Motil. 2011;17(4):338–348.
- Michalakis K, le Roux C. Gut hormones and leptin: impact on energy control and changes after bariatric surgery—what the future holds. Obes Surg. 2012; 22:1648–1657.
- Korczala-Zwirska K, Konturek SJ, Sodowski M, et al. Basal and postprandial plasma levels of PYY, ghrelin, cholecystokinin, gastrin and insulin in women with moderate and morbid obesity and metabolic syndrome. J Physiol Pharmacol. 2007;58:Suppl 1:13–35.
- Dimitriadis E, Daskalakis M, Kampa M, et al. Alterations in gut hormones after laparoscopic sleeve gastrectomy: a prospective clinical and laboratory investigational study. Ann Surg. 2013; 257:647–654.
- Terra X, Auguet T, Guiu-Jurado E, et al. Long-term changes in leptin, chemerin and ghrelin levels following different bariatric surgery procedures: Roux-en-Y gastric bypass and sleeve gastrectomy. Obes Surg. 2013;23(11):1790-8.
- le Roux CW, Aylwin SJ, Batterham RL, et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006; 243:108–114.
- Reinehr T, Roth CL, Schernthaner GH, et al. Peptide YY and glucagon-like peptide-1 in morbidly obese patients before and after surgically induced weight loss. Obes Surg. 2007; 17:1571–1577.
Category: Past Articles, Review







