Colonic Interposition Graft as a Conduit following Esophagectomy for Adenocarcinoma in the Setting of Sleeve Gastrectomy
by Salim Abunnaja, MD, FACS, FASMBS; Daniel Sprando, MD; Jad M. Abdelsattar, MD; Reima El Naili, MD; and Ghulam Abbas, MD, MHCM, FACS
All authors are with the Department of Surgery, West Virginia University in Morgantown, West Virginia.
Funding: No funding was provided for this article.
Disclosures: The authors have no conflicts of interest relevant to this article.
Bariatric Times. 2022;19(12):16–18.
Abstract
The prevalence of obesity has reached epidemic proportions. Worldwide, nearly 580,000 people undergo bariatric surgery annually, with sleeve gastrectomy being the most commonly performed procedure. Despite the effectiveness of sleeve gastrectomy in treating obesity, gastroesophageal reflux and its related complications, such as Barrett’s esophagus and esophageal adenocarcinoma, remain an area of concern. In addition, the surgical management of esophageal cancer following sleeve gastrectomy is much more complex due to the alteration of gastric anatomy and reserve. We discuss in this article the feasibility and technique of colon interposition in the setting of sleeve gastrectomy. To our knowledge, this has never been described in the literature. The article may help familiarize surgeons faced with such a situation in the future, given the significant rise in the popularity of sleeve gastrectomy as a treatment for obesity worldwide.
Keywords: Gastric sleeve, gastroesophageal reflux disease, colonic interposition, esophagectomy
The prevalence of obesity has reached epidemic proportions worldwide and has been identified as a risk factor for numerous medical conditions including, gastroesophageal reflux disease (GERD).1 Patients with longstanding GERD are at increased risk for Barrett’s esophagus (BE), which predisposes patients to esophageal adenocarcinoma.1 It is now widely accepted that bariatric surgery is the most effective therapy combating obesity and its harmful sequela. Although many consider Roux-en-Y gastric bypass (RYGB) surgery to be the gold standard bariatric procedure, laparoscopic sleeve gastrectomy (SG) has emerged as a comparable alternative, often favored due to its technical simplicity.1
A detriment of SG is that it can worsen or create de novo GERD. This is due to the increase in luminal gastric pressure that arises from decreasing total gastric volume.2 Although SG has become the most common bariatric operation performed in the United States (US), there is a paucity of data regarding the incidence of BE and esophageal adenocarcinoma associated with increased GERD in patients who undergo SG.2
There are currently only a handful of case reports of patients with SGs who have either developed BE or esophageal adenocarcinoma and no reports of subsequent surgical therapy in the setting of SG. Surgery in patients who have had a SG is more complex due to the alteration of gastric anatomy and reserve.1,2 Typically the first choice of conduit when performing esophagectomy for adenocarcinoma, the stomach is no longer an option for reconstruction in patients who undergo esophagectomy. Herein, we discuss the management of esophageal adenocarcinoma that developed in a patient who had undergone laparoscopic SG, which was ultimately surgically managed with esophagectomy and reconstruction using a colonic interposition graft.
Case Presentation
A 58-year-old female patient presented with a history of mild progressive dysphagia to solid food over several months and unintentional 20-pound weight loss. Her past medical history was significant for GERD for 15 years, controlled with proton pump inhibitor therapy. She was a smoker and a social drinker. Her surgical history was significant for a laparoscopic SG performed seven years prior to presentation for Class III obesity. She had excellent weight loss results from her SG with resolution of her Type 2 diabetes mellitus, hypertension, and obstructive sleep apnea. In addition, she had a remote history of a total abdominal hysterectomy with bilateral salpingo-oophorectomy for Stage IA serous borderline ovarian tumor. Her family history was notable for lung cancer in her mother.
Physical exam was unremarkable, with no palpable lymphadenopathy. Workup including an esophagogram revealed a distal esophageal stricture. An upper endoscopy revealed a partially obstructing circumferential fungating mass arising from the background BE. Staging computed tomography (CT) and positron emission tomography (PET) scans ruled out metastatic disease and mediastinal lymphadenopathy (Figure 1).
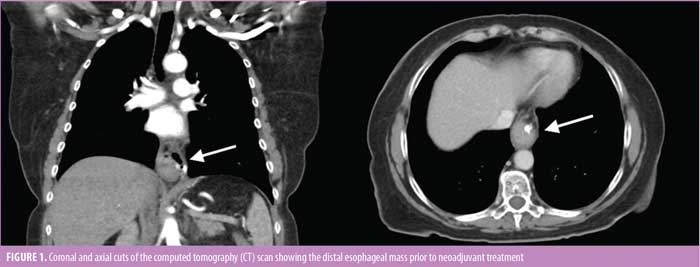
A magnetic resonance imaging (MRI) scan of the brain showed no evidence of metastasis. Endoscopic ultrasound (EUS) demonstrated invasion into the adventitia, and biopsy revealed poorly differentiated adenocarcinoma (human epidermal growth factor receptor 2 [HER2/neu]-negative and programmed death-ligand 1 [PDL1]- positive). The tumor was staged T3N0M0.
The patient was treated with neoadjuvant chemoradiation as per CROSS Trial using paclitaxel and carboplating with 50.4Gy of radiation. She had a good response to her chemoradiation, and preoperative planning was started. In the absence of a suitable gastric conduit, given her history of SG, it was decided to proceed with esophagectomy with colon interposition. A preoperative colonoscopy revealed no colonic pathology and CT angiography of the abdomen showed normal colon vasculature.
Technique Description
After induction of general anesthesia and a bronchoscopic and endoscopic evaluation, a midline laparotomy was performed. The case started by assessing and preparing the colon conduit. Both the hepatic and splenic flexures were mobilized, and the greater omentum was excised. The vascularity of the colon was assessed and confirmed by identifying and isolating the ileocolic, right colic, middle colic, and left colic vessels. Adjuncts used to help assess bowel vascularity included transillumination, indocyanine green (ICG) perfusion angiography, and temporary clamping of the vessels. The right colon was chosen as the conduit (Figure 2).
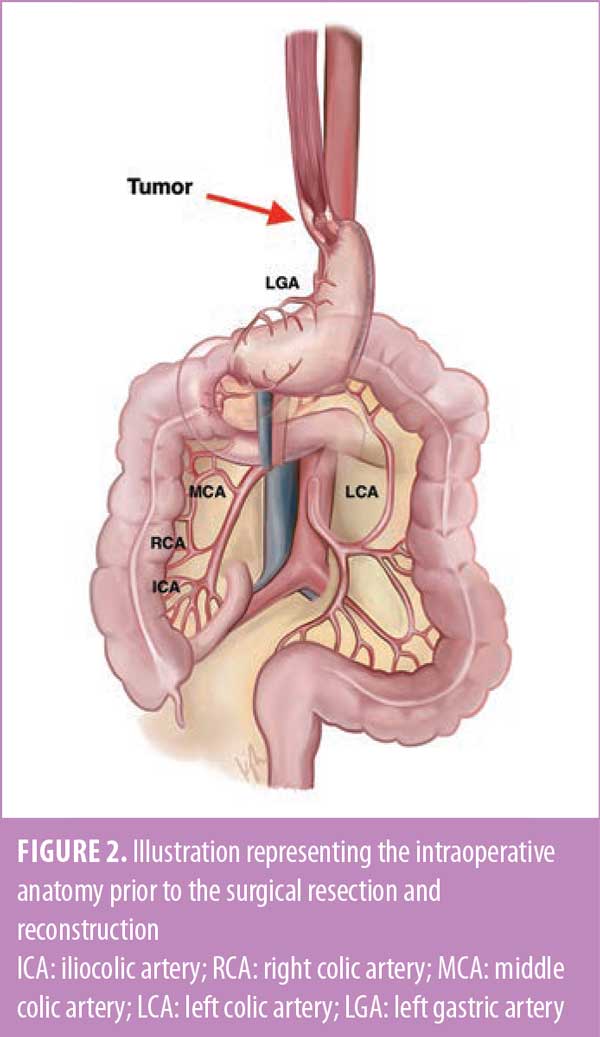
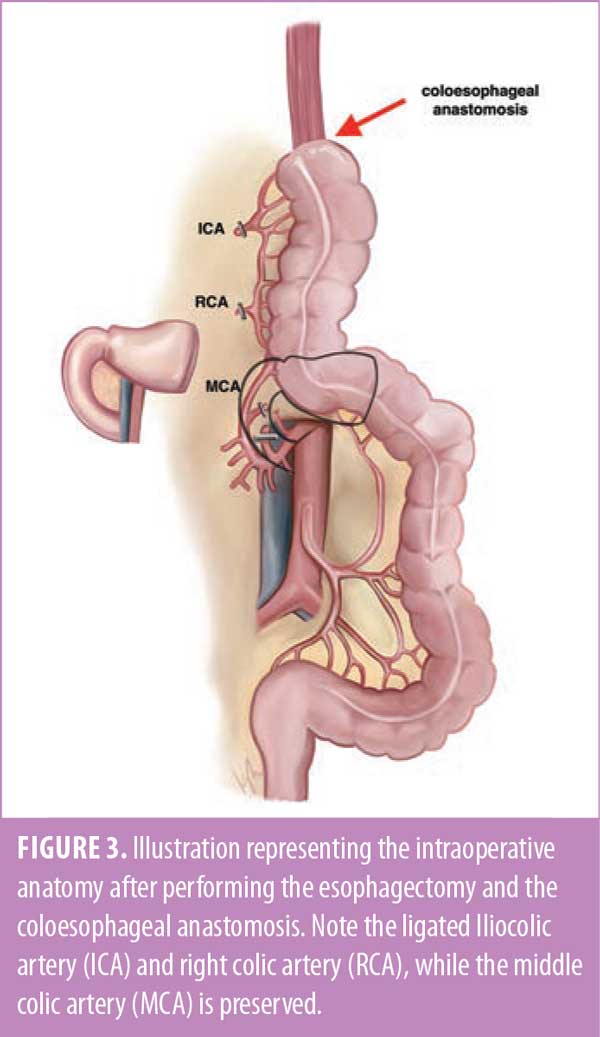
A transhiatal esophagectomy was performed by mobilizing the intra-abdominal portion of the esophagus, dissecting the esophagus circumferentially and cephalad into the mediastinum. Dissection of the
esophagus in the neck was facilitated by a left cervical incision along the anterior border of the sternocleidomastoid muscle. The esophagus was then transected and pulled down into the abdomen, and the proximal part of the sleeved stomach was transected while preserving the antrum and its blood supply. The specimen was handed over. The hiatus was closed. A retrosternal tunnel was created for the colon conduit, and the left sternoclavicular joint was excised to avoid undue compression.
The right colon was mobilized, the terminal ileum was transected, and both the ileocolic and right colic vessels were ligated and divided. The middle colic and marginal vessels were preserved. The right colon was then brought up through the substernal tunnel and delivered into the cervical incision without tension, which facilitated formation of a coloesophageal anastomosis with a linear stapler (Figure 3).
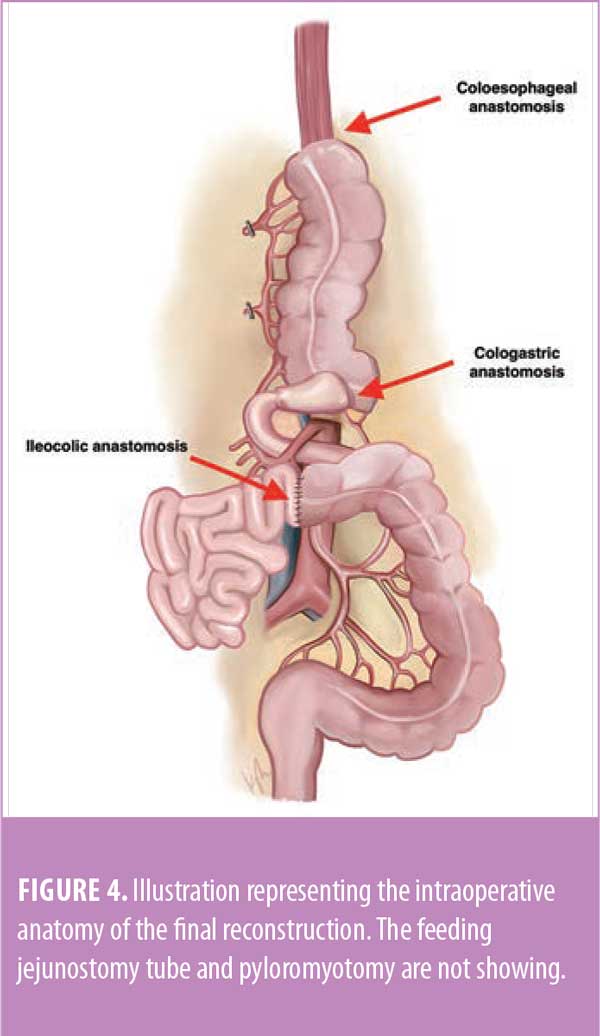
Distally, the transverse colon was transected after the redundancy was pulled out of the chest, and a side-to-end cologastric anastomosis with the antrum was created using a linear stapler. To complete the reconstruction, the ileum was anastomosed to distal transverse colon in a side-to-side functional end-to-end manner using a linear stapler. Figure 4 shows the final reconstruction.
Finally, a pyloromyotomy was performed to ensure adequate drainage of the antrum, a jejunostomy feeding tube was placed to ensure adequate postoperative nutrition, and bilateral chest tubes were placed for pleural drainage.
Postoperative Course
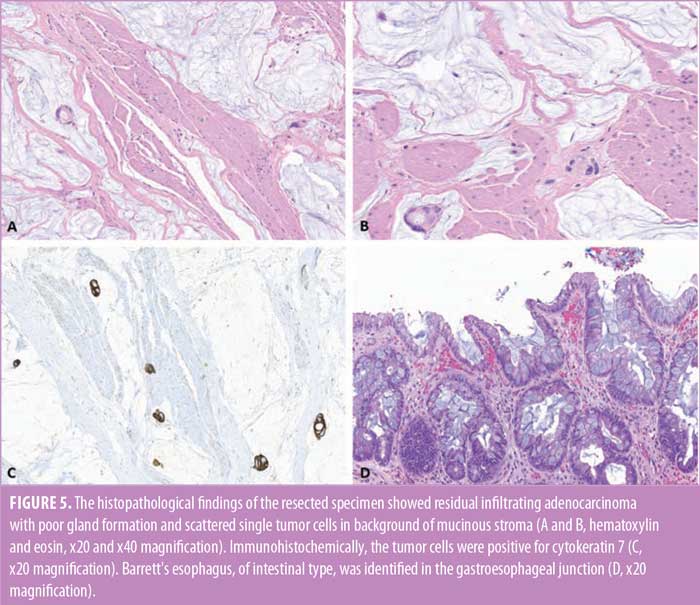
Postoperative course was complicated by development of a hoarse voice and left vocal cord paralysis. While nutrition was maintained via tube feeds through her jejunostomy tube, her upper gastrointestinal (GI) study performed on postoperative Day 5 demonstrated a leak at the coloesophageal anastomosis. She remained hemodynamically stable without signs of sepsis, and the leak was managed conservatively. She was eventually discharged home, and her diet was advanced as tolerated. The feeding jejunostomy tube was removed one month postoperatively, once she was able to meet her caloric needs orally. The final pathological findings of the posttreatment resected specimen revealed a residual, poorly differentiated adenocarcinoma, infiltrating the adventitial layer of the esophagus. A partial response to preoperative treatment was evident in the resected specimen (tumor regression score 2). Immunohistochemically, the tumor cells were positive for cytokeratin. The surgical margins were cancer-free, and all the submitted regional lymph nodes were free of metastatic carcinoma (Figures 5A–5D).
Currently, the patient is over one-year postsurgery. She remains in remission, tolerating a regular diet, and is maintaining her weight. However, she had two sessions of upper endoscopy with dilation of the coloesophageal anastomosis for mild dysphagia.
Discussion
Although it has been established that longstanding GERD and BE are risk factors for the development of esophageal adenocarcinoma, the exact risk of esophageal adenocarcinoma after SG has not been established. SG is an effective therapy for obesity but is a potential risk factor for esophageal adenocarcinoma, as it can worsen or create de novo GERD, with some studies demonstrating increased incidence of BE in both symptomatic and asymptomatic patients.2 The association between SG and esophageal cancer, however, has not been established in the current literature, save for a few published case reports.2 If, indeed, an increased risk of esophageal adenocarcinoma is found in patients who undergo SG, the role and frequency of postoperative screening and surveillance should be examined.
Current clinical guidelines state that esophagogastroduodenoscopy (EGD) is indicated for GERD refractory to medical management, in the presence of alarm symptoms (dysphagia, weight loss, hematemesis), patients with multiple risk factors for BE (age >50 years, Caucasian race, male sex, family history of BE or esophageal adenocarcinoma, prolonged GERD, smoking and/or obesity), and patients with established diagnosis of BE. The American Society for Gastrointestinal Endoscopy recommends EGD screening for patients with established BE diagnosis every 3 to 5 years.3 The reality is that patients who present with alarming symptoms have often already developed esophageal adenocarcinoma, as is the case with the patient presented herein. This is often an advanced disease that requires neoadjuvant chemoradiation, followed by esophagectomy in surgical resectable cases, which confers superior five-year survival.
The role of EGD in patients being evaluated for bariatric surgery is somewhat elusive. The International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) examined the role of EGD in pre- and postoperative bariatric surgery patients. Their recommendation is that EGD should be considered for all patients with or without GI symptoms prior to surgery. This is because 25.3 percent of asymptomatic individuals have abnormal pathology that might alter surgical management or be a contraindication to surgery.4 For example, patients who were found on preoperative endoscopy to have GERD esophagitis or BE are better served with RYGB than SG. RYGB is an effective bariatric, as well as antireflux, surgical procedure, treating both obesity and GERD. In addition, both endoscopic and histologic regression of BE can be seen after the procedure.5
For all patients, EGD should be undertaken routinely after bariatric surgery at one year, then every 2 to 3 years for patients who have undergone SG or one anastomosis gastric bypass. This is for the early detection of BE or GI malignancies. For all other types of bariatric surgical patients, postoperative EGD is indicated on the basis of GI symptoms, as previously mentioned.4 The ultimate goal is for earlier detection of lower stage disease or premalignant dysplasia, which confers superior survival and allows for less invasive therapies, such as endoscopic mucosal resection and ablation, when appropriate.
The opportunity to utilize less invasive/radical therapies is particularly important in patients who undergo SG. This is because the sleeved stomach cannot be used as a gastric conduit for reconstruction following esophagectomy given the altered gastric anatomy and the absence of the gastroepiploic blood supply.6 As demonstrated by this case report, surgical management of invasive esophageal adenocarcinoma in patients with SG can be complex and highlights the importance of establishing strict screening and surveillance guidelines. Surgeons should also be familiar with the complexity and options of reconstruction in this patient subset, such as the colonic interposition reconstruction.
Although the use of a colonic interposition graft for reconstruction following esophagectomy is a well-described option, the associated postoperative mortality has been shown to be somewhat higher than that with gastric conduit, ranging from 10 to 30 percent. Morbidity and mortality stems from graft necrosis, which results from injury to the vessels supplying the graft by clamps or energy devices, or through excessive torsion and traction. Furthermore, anastomotic leaks can occur in up to 10 percent of patients following esophagectomy with colonic interposition graft reconstruction. Some patients develop dysphagia, graft fibrosis, reflux colitis, and intrinsic colonic pathology, such as inflammatory bowel disease and colon cancer.6
Colonic interposition can be performed using the right colon, as in this case, with an isoperistaltic configuration, or the left colon, using either isoperistaltic or antiperistaltic configurations. When using the right colon for reconstruction, the ascending colon is mobilized and transected at the terminal ileum and is brought to the proximally resected esophagus where an anastomosis is created. The transverse colon is transected and the graft segment is re-anastomosed to the remaining gastric pouch, or jejunum if the stomach is completely resected. Finally, the ileum is anastomosed to the distal transverse colon. Peristaltic colonic contractions occur less frequently than enteric contractions and can be advantageous for water and nutrient absorption. Another advantage of using a colon interposition graft is that it can serve as an antireflux mechanism in patients with a competent ileocecal valve.6
When utilizing the left colon as an interposition graft, both the left and right colon are mobilized, and the transverse colon is divided. The left colon is brought to the proximal esophagus to form the proximal anastomosis. The distal portion of the graft is formed by dividing the left colon and anastomosing the distal portion of the graft with either the jejunum or stomach. Colonic continuity is reestablished with a colo-colonic anastomosis of the intra-abdominal right and left colon. Alternatively, the left colonic interposition graft can be created with an antiperistaltic configuration. This is accomplished by dividing the left colon distally (often including the sigmoid colon), which can provide greater length for the conduit if needed.6
Conclusion
Regardless of the reconstruction method utilized, it is important to have surgeons who are familiar with colonic interposition techniques, especially when the the popularity of bariatric surgery in treating obesity is rising and the altered gastric anatomy might be a potential risk of developing esophageal cancer.
Statement of Consent
Informed consent was obtained for the use of deidentified patient date for publication.
Author Contributions
Salim Abunnaja and Ghulam Abbas performed the surgical operation and provided the clinical care to the patient, in addition to writing the case presentation and surgical technique. Daniel Sprando and Jad M. Abdelsattar were responsible for the literature review, illustrations, and manuscript preparation. Reima El Naili prepared the pathology section and provided intellectual contribution to study design.
References
- El Khoury L, Benvenga R, Romero R, et al. Esophageal adenocarcinoma in Barrett’s esophagus after sleeve gastrectomy: case report and literature review. Int J Surg Case Rep. 2018;52:132–136.
- Qumseya BJ, Qumsiyeh Y, Ponniah SA, et al. Barrett’s esophagus after sleeve gastrectomy: a systematic review and meta-analysis. Gastrointest Endosc. 2021;93(2):343–352.e2.
- Muthusamy VR, Lightdale JR, Acosta RD, et al. The role of endoscopy in the management of GERD. Gastrointest Endosc. 2015;81(6):1305–1310.
- Brown WA, Johari Halim Shah Y, Balalis G, et al. IFSO position statement on the role of esophago-gastro-duodenal endoscopy prior to and after bariatric and metabolic surgery procedures. Obes Surg. 2020;30(8):3135–3153.
- Andrew B, Alley JB, Aguilar CE, Fanelli RD. Barrett’s esophagus before and after Roux-en-Y gastric bypass for severe obesity. Surg Endosc. 2018;32(2):930–936.
- Depypere L, Van Veer H, Nafteux PR, et al. (2019). Options for esophageal replacement. In: Yeo C, Shackelford’s Surgery of the Alimentary Tract, 2 Volume Set. Eighth edition. Elsevier; 2019:438–466.
Category: Case Report, Past Articles




