Applying Metabolic Surgery Research to the Type 1 Diabetes Treatment Algorithm: A Novel Hypothesis
An interview with:
Esteban Varela, MD, FACS, FASMBS
Professor of Surgery at University of Central Florida and Chair of Surgery at HCA Medical Center, Orlando, Florida, and Diabetes Surgery Institute, Bogota, Colombia
Carlos Felipe Chaux, MD
Bariatric Surgeon and Chief at Cirugia para la Obesidad, Bogota, Colombia, and Diabetes Surgery Institute, Bogota, Colombia
In a recent article published in CellR4,[1] Chaux et al proposed a novel hypothesis of utilizing metabolic surgery in combination with adult stem cell therapy to promote β-cell regeneration, which might result in insulin independence in type 1 diabetes mellitus. They discuss how intestinal reconstructions in metabolic surgery, which have proven effective in the treatment of patients with type 2 diabetes mellitus, could be extrapolated to type 1 diabetes mellitus. They concluded that metabolic surgery and stem cells should be considered as part of a multimodal treatment algorithm for type 1 diabetes mellitus treatments, and propose a protocol requiring a multidisciplinary team approach and infrastructure including metabolic surgeons, endocrinologists, immunologists, and regenerative medicine specialists. Bariatric Times interviewed Drs. Esteban Varela and Carlos Felipe Chaux to learn more about this new area of research.
Funding: No funding was provided in the preparation of this manuscript.
Financial disclosures: The authors report no conflicts of interest relevant to the content of this article.
Bariatric Times. 2017;14(7):22–23.
Please explain the pathophysiology of both types 1 and 2 diabetes mellitus. How do they differ? Please explain the pathophysiology of both types 1 and 2 diabetes mellitus. How do they differ?
Drs. Varela & Chaux: Diabetes varies on its pathophysiology and age of presentation. In general, type 1 diabetes mellitus (T1DM) presents in childhood as an autoimmune process that destroys beta cells, resulting in life-long insulin-dependence. On the other hand, type 2 diabetes mellitus (T2DM) is of adult onset and mostly related to obesity, lipotoxicity, pancreas burnout, hyperinsulinemia, peripheral insulin resistance, and eventually insulin dependence. In addition, there is an uncommon presentation in the adulthood that is also autoimmune mediated, so called latent autoimmune diabetes in adults (LADA).
In the article you state the following, “It is also possible that a small percentage of obese patients are mislabeled as T2DM when in reality they belong to the T1DM spectrum, with associated severe obesity and may present with clinical manifestations of both diabetes types (i.e., auto-antibodies, insulin resistance, and insulin dependence).” How would “mislabeling” affect their treatment?
Drs. Varela & Chaux: It is possible that a very small percentage of patients with LADA are being labeled and treated as insulin dependent type 2 diabetics without knowing their immune status (i.e., autoantibodies titers). This small subset of patients might not be good responders to bariatric surgery. Nevertheless, surgical treatment should be the same for both groups. As it has been previously shown, subject with obesity with T1DM show minimal improvements of their glycemic profile and insulin requirements up to 50 percent.
When and why did you and your colleagues begin to hypothesize that metabolic surgery in combination with adult stem cell therapy to promote β-cell regeneration may result in insulin independence in T1DM?
Drs. Varela & Chaux: We as bariatric and metabolic surgeons already offer the best treatment for T2DM, which provides effective and long-term remission of this condition when compared to medical therapy alone.[2] However, we are running short treating T1DM with bariatric surgery. Although there are some positive metabolic responses after surgery, a complete remission has not been achieved. The answer might seem intuitive as there are different pathophysiologic entities, and there is completely destruction of pancreatic islet cells (Figure 1). However, there is evidence that even individuals with T1DM have dormant beta cells that might be reactivated when appropriately stimulated. Stem cell therapy may provide and promote pancreatic cell regeneration.
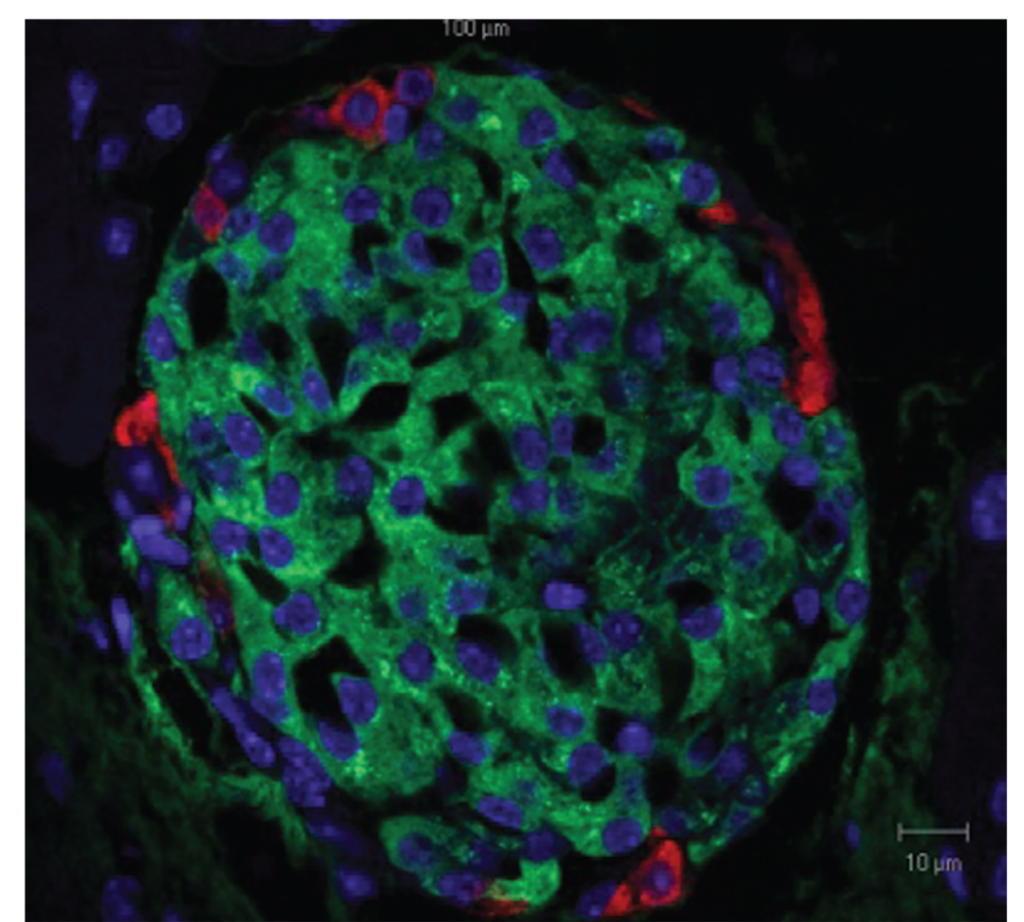
Figure 1. Pancreatic islet as seen by light microscopy. Beta cells can be recognized by the green insulin staining. Glucagon is labeled in red and the nuclei in blue.Copyright Nepton S. Beta-cell function and failure. In: Escher A (ed.) Type 1 Diabetes, Rijeka, Croatia: InTech; 2013.
Has the utilization of metabolic surgery in conjunction with implantation of adult stem cells been studied previously?
Drs. Varela & Chaux: Stem cells from various sources have been previously studied by Shapiro et al[3] for the treatment for T1DM. However, the utilization of stem cells after bariatric surgery is a new hypothesis that, to date, has not been tested.
What are the mechanisms of action in utilizing this therapy in treating T1DM?
Drs. Varela & Chaux: Explanations from the paper: An elongated biliopancreatic (BP) limb reconstruction may be able to further augment this incretin activity and β-cell trophic factors, mainly by glucagon-like peptide 1 (GLP-1), gastric inhibitory polypeptide (GIP), and peptide (PYY) effects. Prior rodent studies by Kamvissi et al showed the importance of the BP limb in diabetes remission and its positive incretin effects.[4] Typical teaching during Roux-en-Y gastric bypass (RYGB) reconstruction (Figure 2) has been to perform BP limbs between 30 and 50cm in length. We have advocated longer BP limbs up to 150cm for patients with diabetes to maximize incretin activity with no observed adverse absorptive effects (i.e., a 150/150cm Roux/BP limb reconstruction).[5] This elongated BP limb practice during RYGB is being popularized in Europe and Brazil.
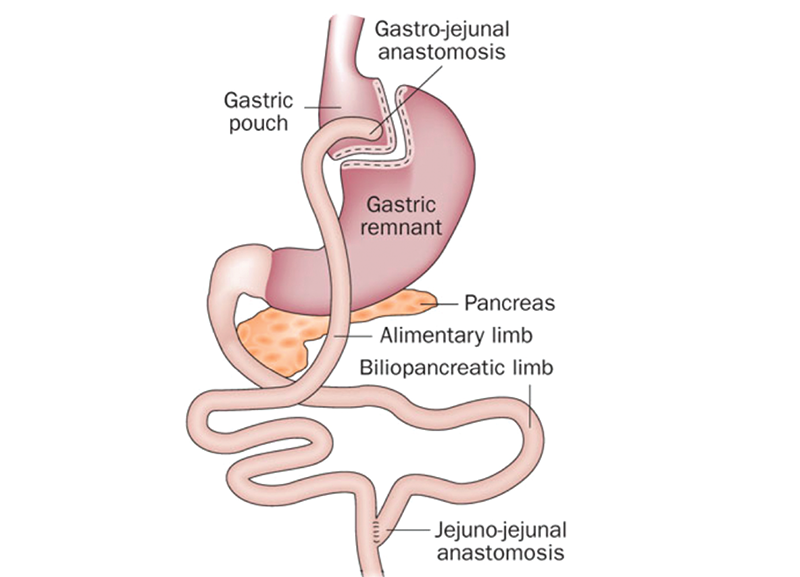
Figure 2. Roux-en-Y gastric bypass reconstruction
What procedure of stem cell regeneration therapy would be used in this hypothesis?
Drs. Varela & Chaux: We are utilizing adult stem cells as compared to embryonic stem cells. Adults carry a large number of stem cells that are easily accessible in the fat tissue and bone marrow. These stem cells are harvested, processed, and implanted at specific sites and times after RYGB. We have yet to determine the proper cell donor sites and cell preparation procedures.
The Metabolic Surgery plus Stem Cell (METASTEM) protocol will include patients with and without obesity. Six months following metabolic surgery, patients will undergo bone marrow harvesting for stem cell procurement and processing. How might the six-month outcomes of the metabolic surgery affect the stem cell regeneration?
Drs. Varela & Chaux: The majority of patients with T1DM are either normal weight or overweight. Very seldom do they have obesity. We plan to study both diabetic groups. Bariatric surgery provides the best possible metabolic environment (by decreasing lipotoxicity, increasing incretins, and insulin sensitivity) for stem cell implantation and long-term survival. We believe that six months may be the right time for those metabolic changes to take place prior to stem cell implantation. After that time, prepped cells will be laparoscopically implanted at specific organ sites. In addition, adult stem cells, as compared to islet cell transplants, are readily available and less costly, without the need for life-long immunosuppression.
What stage is the METASTEM protocol in currently?
Drs. Varela & Chaux: We are currently conducting pilot studies and searching for additional funding. We envision stem cells being part of the bariatric surgeons’ armamentarium along with lifestyle, pharmacologic, endoscopic microbiology and surgical interventions and as part of a simultaneous multimodal approach.
References
- Chaux F, Torres F, Bolaños E, et al. Metabolic surgery and beta cell regeneration in type-1 diabetes: a novel hypothesis. CellR4. 2016;4(3):e2068
- Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes—5-Year outcomes. N Engl J Med. 2017;376(7):641–651.3. Shapiro AM, Pokrywczynska M, Ricordi C. Nat Rev Endocrinol. 2017;13(5):268–277.
- Kamvissi V, Salerno A, Bornstein SR, Mingrone G, Rubino F. Incretins or anti-incretins? A new model for the “enteropancreatic axis.” Horm Metab Res. 2015;47: 84–87.
- Chaux F, Bolaños, E, Varela E. Lengthening of the biliopancreatic limb is a key step during revisional Roux-Y gastric bypass for weight regain and diabetes recurrence. Surg Obes Relat Dis. 2015;11(6):1411.
Can the foregut and hindgut theories have a synergistic effect? Commentary to manuscript: “Metabolic surgery and beta cell regeneration in type 1 diabetes:
a novel hypothesis”
by Raul J. Rosenthal, MD, FACS, FASMBS
Clinical Editor, Bariatric Times; Professor of Surgery and Chairman, Department of General Surgery; Director of Minimally Invasive Surgery and The Bariatric and Metabolic Institute; General Surgery Residency Program Director; and Director, Fellowship in MIS and Bariatric Surgery, Cleveland Clinic Florida, Weston, Florida
Reprinted with permission from: CellR4. 2016;4(3):e2075
I commend the authors for their innovative concept of combining metabolic surgery with stem cell transplantation in order to treat type 1 diabetes mellitus. The hypothesis of this treatment modality is based on creating a more physiologic environment for transplanted adult stem cells to engraft and better function by allowing patients to lose weight and decrease insulin resistance and insulin levels by means of a metabolic procedure such as the gastric bypass. The authors contend that in addition to the above-mentioned benefits, this procedure will bypass the duodenum and proximal jejunum enhancing incretins and anti-incretin effects.1 I would add to this hypothesis the potential effects and benefits of rapid emptying and transit time seen in patients undergoing gastric bypass. The latter will deliver undigested food to the ileum stimulating the L-Cells to the release of glucagon-like peptide 1 (GLP-1) and other gut hormones that might in turn not only further decrease insulin resistance, but also act as a growth factor enabling the transplanted stem cells to engraft better, differentiate, and function.1 I very much look forward to the results of future clinical trials.
References
1. De Graaf C, Blom WA, Smeets PA, Stafleu A, Hendriks HF. Biomarkers of satiation and satiety. Am J Clin Nutr. 2004;79:946–961.
Category: Interviews, Past Articles




