Dysphagia Lusoria After Weight Loss Surgery

by Jeremy Bryner, DO; Erica Ibendahl, MD; and Nicole Fearing, MD, FACS, FASMBS
Drs. Bryner and Fearing are with Menorah Medical Center, Department of Surgery in Overland Park, Kansas. Dr. Ibendahl is with Washington County Hospital, Department of Surgery in Nashville, Illinois.
Funding: No funding was provided for this article.
Disclosures: The authors have no conflicts of interest relevant to this article.
Bariatric Times. 2022;19(4):10–11.
Abstract
Dysphagia lusoria is an impairment of swallowing due to esophageal compression from an aberrant right subclavian artery (ARSA). The majority of patients with ARSA are asymptomatic with incidental discovery on imaging or postmortem analysis. However, 20 to 40 percent of aberrant arteries lead to tracheal-esophageal symptoms such as dysphagia. Currently, the best method for diagnosing dysphagia lusoria is a barium esophagram followed by either a computed tomography (CT) or magnetic resonance imaging (MRI) scan. The severity of symptoms primarily dictates the management of patients with dysphagia lusoria. Often, changes in lifestyle and dietary modification are successful treatments for mild-to-moderate symptoms. However, surgical intervention is justified for patients that do not respond to conservative therapy. We present a 49-year-old female patient who was diagnosed with dysphagia lusoria two months after an uneventful laparoscopic Roux-en-Y divided gastric bypass with primary hiatal hernia repair. After diagnosis, the patient saw a vascular surgeon and underwent plug embolization of her ARSA, followed by right subclavian carotid transposition. Following this procedure, her dysphagia symptoms resolved, she continued to lose weight from her gastric bypass surgery, she was able to come off all diabetes and hypertension medications, and her gastroesophageal reflux symptoms resolved. We hypothesize that the patient’s dysphagia lusoria might be related to her considerable weight loss following gastric bypass surgery, and that the loss of the periesophageal fat pad might have aided in esophageal compression by the ARSA.
Keywords: Dysphagia lusoria, Roux-en-Y divided gastric bypass, weight loss surgery, aberrant right subclavian artery (ARSA), dysphagia
Dysphagia lusoria is an impairment of swallowing due to esophageal compression from an aberrant right subclavian artery (ARSA). Dysphagia lusoria was first discovered by David Bayford in 1761 when studying the death of a 62-year-old woman due to “obstructed deglutition.” Bayford determined that the cause of the patient’s dysphagia with esophageal compression was an ARSA.1
An ARSA occurs during embryogenesis, when the fourth vascular arch and right dorsal aorta involute, leaving the seventh intersegmental artery attached to the descending aorta.2 Most commonly, the ARSA crosses between the esophagus and the vertebral column (80% of cases), but it can also run between the esophagus and the trachea (15% of cases) or pass anterior to both the trachea and esophagus (5% of cases).3
The majority of patients with ARSA are asymptomatic, with incidental discovery on imaging or postmortem analysis. However, 20 to 40 percent of aberrant arteries lead to tracheal-esophageal symptoms.4 Dysphagia consistent with a mechanical obstruction is a common presentation for symptomatic adults.5 Symptoms include regurgitation of unchewed food, postprandial bloating, chest pain,2 thoracic pain, coughing, Horner’s syndrome, and “pill esophagitis.”3
Case Report
The patient was a 49-year-old female, with a medical history of obesity, having a body mass index (BMI) of 36kg/m2; diabetes; hypertension; and burning reflux, who was seen in consultation for weight loss surgery. Her preoperative work-up included an upper endoscopy, which showed evidence of esophagitis, diffuse gastritis, bile reflux, and a moderately sized hiatal hernia. She completed a physician-supervised weight loss program, evaluation from a psychiatrist, and evaluation from a dietitian, and she ultimately underwent an uneventful laparoscopic Roux-en-Y divided gastric bypass with primary hiatal hernia repair. On postoperative day one, the barium esophagram showed normal postoperative changes (Figure 1).
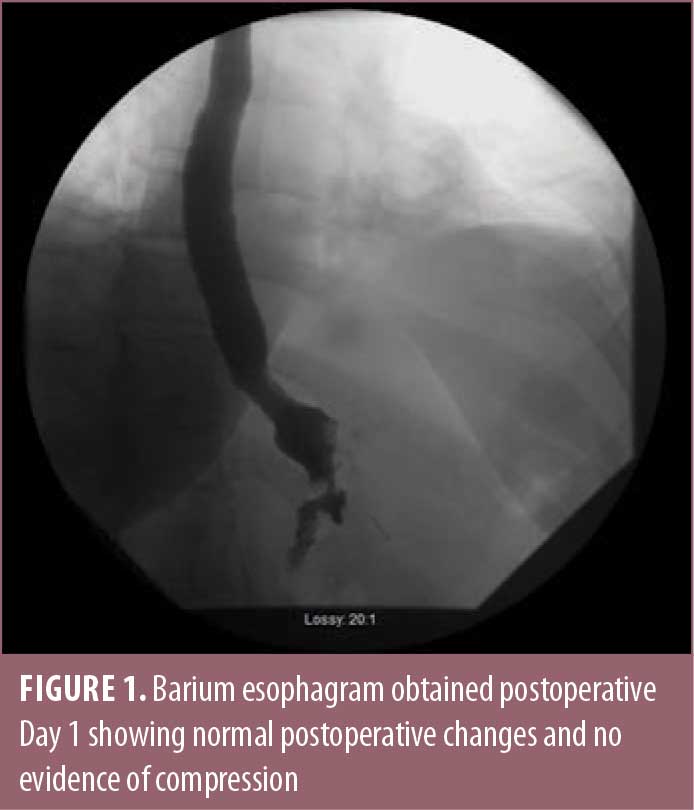
At both one week and one month follow-up, the patient was doing well, tolerating a pureed diet with persistent reflux but no symptoms of dysphagia. At one month postoperative, the patient had lost 17 pounds.
At approximately two months after surgery, the patient began to have symptoms of dysphagia with occasional regurgitation of undigested food. She underwent a computed tomography (CT) scan, as well as an endoscopy, which demonstrated compression of the mid-esophagus by a posteriorly replaced right subclavian artery, consistent with dysphagia lusoria. During the barium esophagram, the barium pill got stuck at the level of compression from her ARSA (Figure 2). Her hiatal hernia repair was intact, with no evidence of obstruction at this site.
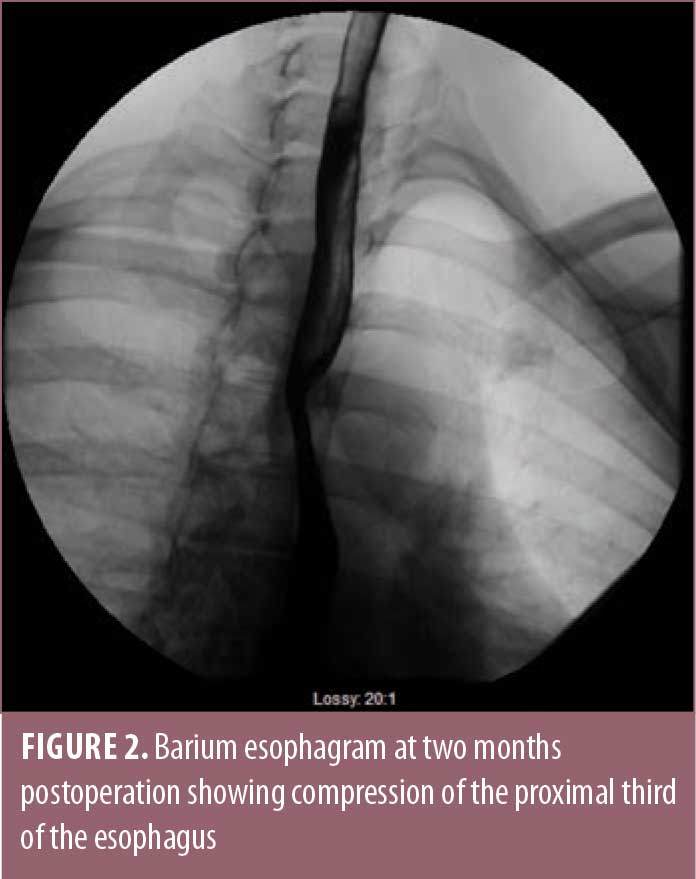
The patient was seen by a vascular surgeon and underwent plug embolization of her ARSA followed by right subclavian to right carotid transposition (Figure 3). Following this procedure, her dysphagia symptoms resolved. She continued to lose weight from her gastric bypass surgery and was able to come off all diabetes and hypertension medications. Her gastroesophageal reflux symptoms completely resolved.
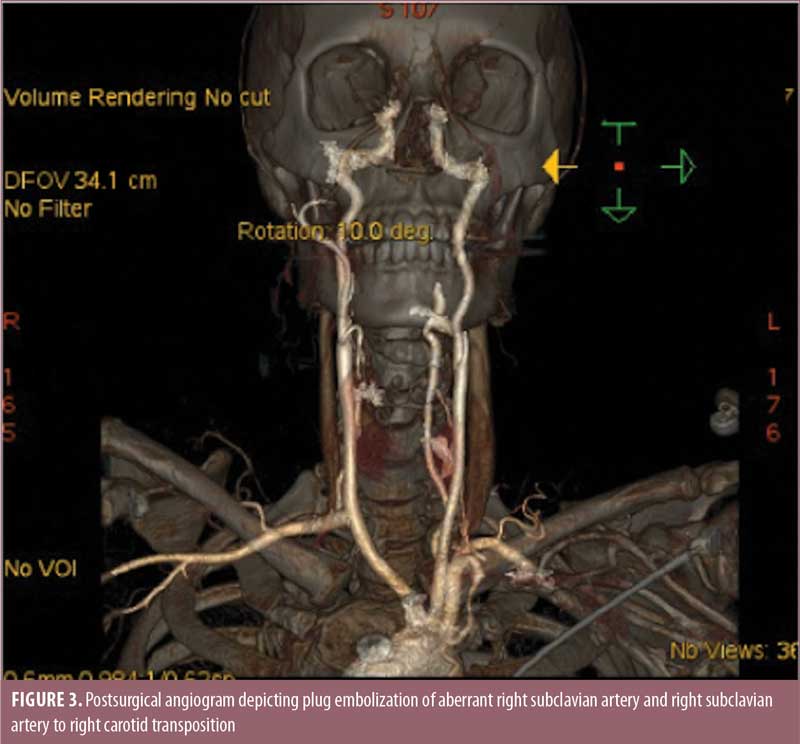
Discussion
Dysphagia lusoria is a rare cause of dysphagia in adults. The majority of patients with dysphagia lusoria present in the fourth decade of life, similar to the patient in this study.
Currently, the best method for diagnosing dysphagia lusoria is a barium esophagram followed by either a CT or magnetic resonance imaging (MRI) scan. An oblique, ascending, extrinsic compression above the level of the aortic arch is typically seen in a barium esophagram conducted on patients with dysphagia lusoria. Both CT angiography and MR angiography can be used to assess the anatomy of the aortic arch and its relationship to surrounding organs.2 The physical exam and upper endoscopy are usually unremarkable.2
The severity of the symptoms primarily dictates the management of patients with dysphagia lusoria. Often changes in lifestyle and dietary modification are successful treatments for mild-to-moderate symptoms.2 Surgical intervention is justified for patients who do not respond to conservative therapy.2
Although surgical management of ARSA has been studied, a specific surgical approach has not been determined.6 Currently, the surgical approach is largely dictated by the surgeon’s personal preferences and the vascular anatomy.2 The common goal of the surgery is to remove the aberrant vessel and to reconstruct the vessel in its appropriate position.2 The literature shows that common surgical approaches include cervical approach, sternotomy, thoracotomy contralateral or ipsilateral to the side of the aortic arch (in cases of aneurysmal disease), or a combined approach.7 In this case report, a cervical approach was utilized using an incision over the supraclavicular fossa.
Conclusion
We hypothesize that the patient’s dysphagia lusoria might be related to her considerable weight loss following gastric bypass surgery, and that the loss of the periesophageal fat pad might have allowed for esophageal compression by the ARSA. A similar case study in which a 23-year-old female patient developed dysphagia five months post-laparoscopic gastric bypass due to an ARSA is reported in the literature.7 As with the patient in our case study, this patient underwent vascular surgery to correct the ARSA and had complete resolution of symptoms.7
References
- Asherson N. David Bayford. His syndrome and sign of dysphagia lusoria. Ann R Coll Surg Engl. 1979;61(1):63–67.
- Levitt B, Richter JE. Dysphagia lusoria: a comprehensive review. Dis Esophagus. 2007;20(6):455–460.
- Chudasama Y, Alcorn J. Dysphagia lusoria: an unexpected sequelum of cardiothoracic surgery. Dig Dis Sci. 2016;61(4):1000–1002.
- Arakoni R, Merrill R, Simon E. Foreign body sensation: a rare case of dysphagia lusoria in a healthy female. Am J Emerg Med. 2018;36(11):213.e1–2134.e2.
- Dandelooy J, Coveliers J, Schil P, Anguille S. Dysphagia lusoria. CMAJ. 2009;181(8):498.
- Estrella M, Chauhan S. Dysphagia lusoria: a rare entity and underrecognized cause of dysphagia. Am J Clin Pathol. 2019;152(Suppl 1):S76–S78.
- Fabian T, Ilves R, Devejian N. Dysphagia lusoria: a complication following gastric bypass surgery? Obes Surg. 2004;14(7):1006–1007.
Category: Case Report, Past Articles



