Successful Endoscopic Closure of a Sleeve Gastrectomy Fistula by an Integrated Multidisciplinary Team: A Case Report
 by Matthew J. Ashbrook, MD, MPH; R. Daniel Lawson, MD; Gordon G. Wisbach, MD, FACS, FASMBS; Jonathan R. Gower, MD; and Kyle D. Gadbois, MD, FACS, FASMBS
by Matthew J. Ashbrook, MD, MPH; R. Daniel Lawson, MD; Gordon G. Wisbach, MD, FACS, FASMBS; Jonathan R. Gower, MD; and Kyle D. Gadbois, MD, FACS, FASMBS
Drs. Ashbrook, Wisbach, Gower, and Gadbois are with the Department of General Surgery, and Drs. Lawson, Wisbach, Gower, and Gadbois are also with the Combined Endoscopy Center, at the Naval Medical Center San Diego in San Diego, California.
FUNDING: No funding was provided.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
Author Disclaimer: The views expressed in this article reflect the results of research conducted by the author and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
Copyright statement: I am a military service member or federal/contracted employee of the United States government. This work was prepared as part of my official duties. Title 17 U.S.C. 105 provides that “copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. 101 defines a U.S. Government work as work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties.
ABSTRACT: The global prevalence of obesity continues to increase, resulting in continued growth of bariatric surgery. Additionally, it is now common for people from the United States to seek bariatric surgery abroad, with laparoscopic sleeve gastrectomy (LSG) being the most frequent bariatric surgery procedure performed. While LSG is a proven and safe method for treating obesity, postoperative leaks are associated with significant morbidity and remain a challenging complication to manage. Although surgical revision has traditionally been a reliable option in the acute postsurgical setting after a leak, endoscopic closure of a staple line leak is now emerging as an effective, definitive management strategy. We present the case of a patient who, after undergoing a LSG, developed a staple line leak and subsequent gastrocutaneous fistula through a port site that was successfully managed endoscopically with an over-the-scope clip, resulting in an expedited recovery and a potential significant decrease in morbidity.
KEYWORDS: Laparoscopic sleeve gastrectomy, obesity, gastrocutaneous fistula, over-the-scope-clip, endoscopy
Bariatric Times. 2021;18(4):14–16.
Bariatric surgery is a safe and effective method for treating morbid obesity, with proven sustained weight reduction and reversal of comorbidities.1,2 With the global burden of obesity increasing in prevalence, there is an expanding presence of bariatric surgery being performed worldwide each year.3,4 Operative procedural costs, medical insurance challenges, domestic wait times, and stringent eligibility criteria for surgery are all motivators that are increasingly driving patients to seek treatment outside the United States (US).5–7 However, there is currently sparse worldwide data on outcomes or postoperative complications, which are infrequently reported on a case-by-case basis within the literature.
Laparoscopic sleeve gastrectomy (LSG) is the most commonly performed bariatric procedure in the US and abroad.4,8 Gastrointestinal (GI) leak rates after LSG have been reported to range from 0 to 7 percent and remain a challenging complication with significant morbidity, mortality, and increased costs due to prolonged hospitalization and intensive care unit (ICU) stays.9,10 Factors associated with increased risk of leak are oxygen dependency, hypertension, diabetes, intraoperative provocative tests, and the placement of a surgical drain.11 Treatment typically requires a multidisciplinary approach involving surgeons, endoscopists, interventional radiologists, and nutrition support to determine the optimal management option.12 Historically, surgical revision has remained the definitive management of LSG leaks.13,14 However, with continual development of novel therapeutic endoscopic device advancements, endoscopic management of leaks is an emerging first-line treatment for safe, reliable, and less invasive alternative to surgery for control or repair of an acute GI perforation.15,16
We present a patient who developed a gastric staple line leak and gastrocutaneous fistula after a LSG that was successfully managed endoscopically using an over-the-scope clip (OTSC).
Case Report and Management
A 46-year-old woman with a past medical history of Class II obesity (body mass index [BMI]=39.2kg/m2) presented to our hospital 16 days after a vertical LSG with a gastric staple line leak. As a result of this leak, she had a 4cm diameter abscess adjacent to the gastric staple line and a gastrocutaneous fistula through an operative-placed laparoscopic 5mm port site incision on the anterior abdominal wall. Unfortunately, the report from the index operation was not available, but according to the patient, her surgery was uncomplicated, despite an intraperitoneal drain placed during that operation. Prior to her initial discharge on postoperative Day 4, the drain was removed after a normal upper GI contrast swallow series, and a clear liquid diet was subsequently started. She was discharged with patient-reported limited postprocedural education, ketorolac, a 10-day course of cephalexin, and no scheduled postoperative follow-up appointment.
On postoperative Day 10, she presented to an urgent care facility for suture removal and reported a persistent thin, brown discharge from the anterior abdominal wall drain site that required twice daily dressing changes. She was discharged home with an extended course of cephalexin, but three days later began experiencing subjective fevers, nausea, vomiting, and abdominal pain prompting her to seek care at the emergency department (ED) at our hospital on postoperative Day 16.
In the ED, the patient was tachycardic but was afebrile with a normal blood pressure. Her abdominal exam was significant for a left upper quadrant abdominal wound at the location of the former drain site that appeared dehisced with serous drainage. Her laboratory studies were remarkable for an increased leukocyte count of 12.4 cells/mL. A computed tomography (CT) abdomen/pelvis with intravenous (IV) and oral
(PO) contrast was performed. This showed a rim-enhancing, multi-loculated fluid collection, containing both enteric contrast and air, with the largest single pocket measuring 4.1cm x 3.9cm x 3.8cm (AP x TR x CC) along the posterior aspect of the greater curvature staple line, just beyond the gastroesophageal (GE) junction (Figure 1). Additionally, the imaging provided evidence that the leak was contiguous with a fistula tract to the anterior abdominal wall at the prior drain site. Importantly, there was no evidence of distal stricture or obstruction on the CT imaging. Her history on presentation had suggested the possibility of a stricture or obstruction distal to the gastric staple line leak with her complaints of nausea and vomiting, but further questioning revealed that she mainly attributed these symptoms to her abdominal pain. Furthermore, she stated that she had not had any difficulty otherwise with tolerating oral intake over the past 16 days. Her laboratory values suggested no evidence of dehydration or acute kidney injury.
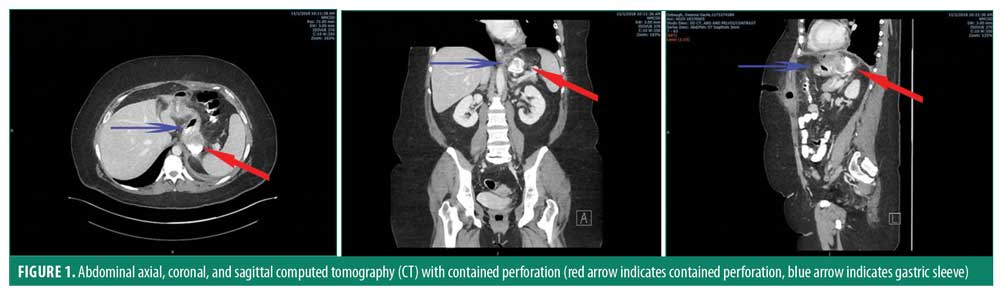
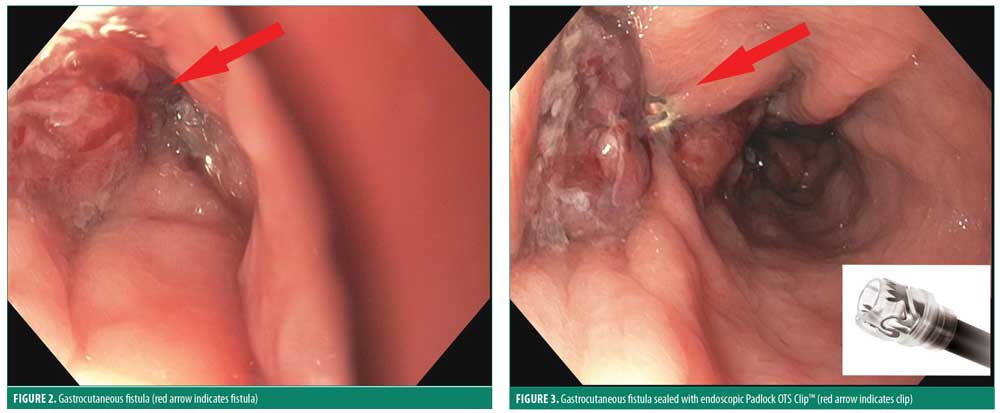
The patient was subsequently admitted to our inpatient metabolic and bariatric surgery service. Her initial care at our institution included nothing by mouht (NPO) status, IV fluids and antibiotics. Gastroenterology was consulted for possible endoscopic closure. Upper endoscopy revealed a 3mm perforation within the gastric sleeve 2cm distal to the GE junction (Figure 2). There was no evidence on endoscopy of distal stricture or obstruction. The staple line dehiscence was surrounded by erythematous and mildly edematous tissue, which exuded a copious amount of purulent material with endoscopic suctioning. A 19mm diameter Padlock OTS clip™ (US Endoscopy, Mentor, Ohio) was deployed to close the defect (Figure 3). A post-pyloric 18 French Dobhoff feeding tube was placed under direct visualization with endoscopic assistance. The patient tolerated the procedure well without complication and subsequently underwent an interventional radiology (IR) guided drain placement in the abscess through the fistula tract. After the successful percutaneous, intraperitoneal, abscess cavity drain placement by IR, post-pyloric tube feeding via the Dobhoff tube was initiated.
Three days later, on postoperative Day 20, the patient was clinically doing well and an interval, planned CT abdomen/pelvis with PO and IV contrast was repeated that showed no evidence of a leak and coaptation of the abscess. With clinical and imaging evidence of complete resolution of the leak after endoscopic OTSC placement, the patient was advanced to an oral liquid and protein bariatric diet while postpyloric, supplemental nutrition via feeding tube was continued.
On postoperative Day 22, six days after OTSC placement, the patient continued to tolerate the liquid and soft food bariatric diet and the anterior abdominal wall drain showed minimal serosanguinous output. The feeding tube was therefore removed and antibiotics were discontinued. She was discharged from the hospital the following day with her IR percutaneous fistula tract drain left in place. This drain was ultimately removed two weeks after being discharged from the hospital while in clinic for follow-up. Her course postendoscopic clip placement was uneventful, and she resumed an unrestricted oral diet without clinical evidence of recurrent leak.
Discussion
With the burgeoning worldwide obesity epidemic, bariatric surgery has increasingly been shown to be a reliable and safe weight reduction modality.1–4 Compared to gastric bypass, LSG is less technically challenging with lower complication rates and is thus the most common bariatric procedure performed globally.4,8,9 Gastric staple line leaks might not present immediately after surgery, as is presented in this case report, but might present several days or weeks later. Nevertheless, staple line leaks are one of the most dreaded complications of metabolic and bariatric surgery, because of the high risk of mortality in the acute stage of staple line leaks, or the morbidity associated with chronic complications, such as gastric fistula development. Although staple line leaks after LSG occur in only 0 to 7 percent of cases, the successful management of staple line leaks remains a challenging complication.9,10
Gastrointestinal leaks after LSG tend to occur within the proximal third of the stomach near the GE junction.9 The etiology of leaks may be due to local tissue ischemia and/or mechanical causes. As a result, operative management is useful for debridement and drainage but often fails to successfully close the defect due to the inflammation and poor tissues.17 Endoscopic management of gastrointestinal leaks with the use of fibrin glue, metallic stents, plugs, or clips are increasingly utilized due to their efficacy and safety profile.15,18
A recent multicenter, retrospective cohort demonstrated an overall 73-percent success rate following endoscopic closure of LSG leaks, with over half of patients experiencing leak resolution after the index intervention.19 OTSCs are being increasingly used in bariatric endoscopy and have been shown to be successful in the management of gastric leaks and fistulas.20,21 Several case series have shown OTSC to have an overall success rate of 73 to 90 percent for the primary management of LSG leaks.22–25 Factors associated with failure of endoscopic treatment include acute leaks, loculated fluid collections, intrabdominal sepsis and low-volume centers.19,25 It is therefore evident that the successful management of LSG leaks is dependent on a multidisciplinary team that includes surgeons, advanced endoscopists, interventional radiologists, and dieticians.
Conclusion
This case demonstrates that the use of therapeutic endoscopy is a safe, reliable, and less invasive alternative to surgery for repair of an acute GI perforation and emphasizes the importance of having a fully integrated multidisciplinary endoscopy unit in the management of LSG leaks. As a result of the relationship between our bariatric surgeons and advanced GI endoscopic specialists, the patient was managed nonoperatively, with an uneventful inpatient clinical course and resumption of an oral diet without clinical evidence of recurrent leak. The perpetual development of new technologies and minimally invasive approaches that limit the potentially extensive morbidity and mortality from such dire complications must be embraced. As the surgical and endoscopic fields continue to advance and overlap, their partnership must spur this evolution and cultivate advancements for better patient outcomes through integrative approaches.
References
- Courcoulas AP, Christian NJ, Belle SH, et al. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA. 2013;310(22):2416–2425.
- Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–1737.
- Seidell JC, Halberstadt J. The global burden of obesity and the challenges of prevention. Ann Nutr Metab. 2015;66 Suppl 2:7–12.
- Welbourn R, Hollyman M, Kinsman R, et al. Bariatric surgery worldwide: baseline demographic description and one-tear outcomes from the Fourth IFSO Global Registry Report 2018. Obes Surg. 2019;29(3):782–795.
- Snyder J, Crooks VA. Medical tourism and bariatric surgery: more moral challenges. Am J Bioeth. 2010;10(12):28–30.
- Turner L. Medical tourism: family medicine and international health-related travel. Can Fam Physician. 2007;53(10):1639–1641, 1646–1648.
- Fruhbeck G. Bariatric and metabolic surgery: a shift in eligibility and success criteria. Nat Rev Endocrinol. 2015;11(8):465–477.
- Abraham A, Ikramuddin S, Jahansouz C, et al. Trends in bariatric surgery: procedure selection, revisional surgeries, and readmissions. Obes Surg. 2016;26(7):1371–1377.
- Aurora AR, Khaitan L, Saber AA. Sleeve gastrectomy and the risk of leak: a systematic analysis of 4,888 patients. Surg Endosc. 2012;26(6):1509–1515.
- Bransen J, Gilissen LP, van Rutte PW, Nienhuijs SW. Costs of leaks and bleeding after sleeve gastrectomies. Obes Surg. 2015;25(10):1767–1771.
- Alizadeh RF, Li S, Inaba C, et al. Risk factors for gastrointestinal leak after bariatric surgery: MBASQIP analysis. J Am Coll Surg. 2018;227(1):
135–141. - Praveenraj P, Gomes RM, Kumar S, et al. Management of gastric leaks after laparoscopic sleeve gastrectomy for morbid obesity: A tertiary care experience and design of a management algorithm. J Minim Access Surg. 2016;12(4):
342–349. - Rebibo L, Dhahri A, Regimbeau JM. Laparoscopic management of gastric leak secondary to distal staple line disunion after sleeve gastrectomy. Surg Obes Relat Dis. 2015;11(4):940–941.
- Kim J, Azagury D, Eisenberg D, et al. ASMBS position statement on prevention, detection, and treatment of gastrointestinal leak after gastric bypass and sleeve gastrectomy, including the roles of imaging, surgical exploration, and nonoperative management. Surg Obes Relat Dis. 2015;11(4):
739–748. - Casella G, Soricelli E, Rizzello M, et al. Nonsurgical treatment of staple line leaks after laparoscopic sleeve gastrectomy. Obes Surg. 2009;19(7):
821–826. - Shoar S, Poliakin L, Khorgami Z, et al. Efficacy and safety of the over-the-scope clip (OTSC) system in the management of leak and fistula after laparoscopic sleeve gastrectomy: a systematic review. Obes Surg. 2017;27(9):2410–2418.
- Oshiro T, Kasama K, Umezawa A, et al. Successful management of refractory staple line leakage at the esophagogastric junction after a sleeve gastrectomy using the HANAROSTENT. Obes Surg. 2010;20(4):530–534.
- Sakran N, Goitein D, Raziel A, et al. Gastric leaks after sleeve gastrectomy: a multicenter experience with 2,834 patients. Surg Endosc. 2013;27(1):240–245.
- Smith ZL, Park KH, Llano EM, et al. Outcomes of endoscopic treatment of leaks and fistulae after sleeve gastrectomy: results from a large multicenter U.S. cohort. Surg Obes Relat Dis. 2019;15(6):
850–855. - Repici A, Arezzo A, De Caro G, et al. Clinical experience with a new endoscopic over-the-scope clip system for use in the GI tract. Dig Liver Dis. 2009;41(6):406–410.
- Von Renteln D, Denzer UW, Schachschal G, et al. Endoscopic closure of GI fistulae by using an over-the-scope clip (with videos). Gastrointest Endosc. 2010;72(6):1289–1296.
- Surace M, Mercky P, Demarquay JF, et al. Endoscopic management of GI fistulae with the over-the-scope clip system (with video). Gastrointest Endosc. 2011;74(6):1416–1419.
- Mercky P, Gonzalez JM, Aimore Bonin E, et al. Usefulness of over-the-scope clipping system for closing digestive fistulas. Dig Endosc. 2015;27(1):18–24.
- Haito-Chavez Y, Law JK, Kratt T, et al. International multicenter experience with an over-the-scope clipping device for endoscopic management of GI defects (with video). Gastrointest Endosc. 2014;80(4):610–622.
- Keren D, Eyal O, Sroka G, et al. Over-the-scope clip (OTSC) system for sleeve gastrectomy leaks. Obes Surg. 2015;25(8):1358–1363.
Category: Case Report, Past Articles



