Hematological Considerations after Bariatric Surgery
 This activity has expired.
This activity has expired.
Course Overview
Despite the American Society for Metabolic Surgery (ASMBS) Allied Health Nutritional Guidelines first published in 2008 (Aills L, Blankenship J, Buffington C, et al. ASMBS Allied Health Nutritional Guidelines for the Surgical Weigh Loss Patient. Surg Obes Relat Dis. 2008;4(5 Suppl): S73-108.), vitamin deficiencies following bariatric and metabolic surgery remains significant. This continuing education article addresses the physiological need for iron and assessment of iron deficiency. Also included are preoperative testing and postoperative screening recommendations, postoperative supplementation for deficiency prevention, as well as repletion suggestions.
Course Description
This continuing education course is designed to educate, through independent study, integrated healthcare clinicians who care for the metabolic and bariatric surgical patient population.
Course Objectives
- Upon completion of this program, the participant should be able to do the following:
- Discuss how iron is absorbed in the human body.
- List the deficient nutrients involved in the development of anemia.
- Explain how obesity affects iron hemostasis.
- Describe how iron absorption is affected following sleeve gastrectomy (SG) and Roux-en-Y gastric bypass (RYGB) surgery.
- List the physical signs and symptoms of iron deficiency following bariatric surgery.
- Describe the 2016 American Society for Metabolic and Bariatric Surgery (ASMBS) Guidelines on iron supplementation following adjustable gastric banding, vertical SG, RYGB, and biliopancreatic diversion (BPD) with duodenal switch (DS).
Target Audience
This accredited program is intended for nurses who treat patients with overweight or obesity.
Completion Time
This continuing education activity is accredited for a total of 1.0 contact hour (Nursing).
Provider
This continuing education activity is provided by Matrix Medical Communications. Provider approved by the California Board of Registered Nursing, Provider Number 14887, for 1.0 contact hour.
Provider Contact Information
Amanda Tolvaisa, Matrix Medical Communications, 1595 Paoli Pike, Suite 201, West Chester, PA 19380; Email: atolvaisa@matrixmedcom.com
Sponsorship and Support
This continuing education activity is financially supported by Bariatric Advantage (Aliso Viejo, California).
Tracy Martinez, RN, BSN, CBN
Ms. Martinez is Department Editor of Integrated Health Continuing Education for Bariatric Times; and Program Director of Wittgrove Bariatric Center in Del Mar, California.
A Message from the Department Editor
Dear Colleagues:
Achieving optimal outcomes, both short- and long-term, following bariatric and metabolic surgery should be all team members goal, including the most important team member, the patient. Bariatric surgery is undeniably the most effective treatment for the disease of severe obesity and the associated disease complications, however the potential for vitamin and nutritional deficiencies remains a threatening complication. This risk is avoidable with a comprehensive preoperative assessment, patient education, specific and clear guidance and ongoing monitoring post operatively. This edition’s Integrated Health Continuing Education article provides in-depth education on the hematological consideration following bariatric and metabolic surgery.
I hope you enjoy this comprehensive article and make any necessary clinical changes in your practice. Please share this with your colleagues to help ensure the best outcomes for your patients.
My Best to You,
Tracy Martinez, RN, BSN, CBN
by Cassie I. Story, RDN
Ms. Story has a private practice in Scottsdale, Arizona.
Funding: This continuing education activity was funded by Bariatric Advantage (Aliso Viejo, California)
Disclosures: Cassie I. Story, RDN, is the Director of Nutrition Education for Bariatric Advantage.
Abstract: There is an urgent need for science-based nutrition recommendations for patients who have undergone bariatric surgery. While evidence indicates that bariatric surgery is the most effective treatment option for someone affected by obesity, the increase in reported nutrition deficiencies and complications due to inadequate nutritional status of bariatric patients postoperatively is cause for alarm. In Part 2 of a four-part Core 4 educational series on identifying, preventing, and treating nutrition deficiencies in preoperative and postoperative bariatric surgery patients, we focus on anemia and iron deficiency. In this continuing education article, we review how bariatric surgery can impact iron absorption, discuss pre- and postoperative screening for iron deficiency and iron deficiency anemia, and provide nutrition treatment recommendations for bariatric patients with iron deficiency and/or anemia.
Keywords: bariatric surgery, iron deficiency, iron deficiency anemia
Bariatric Times. 2019;16(12):10–14.
The impact of micronutrient deficiencies on hematological disorders after surgery are extensive. Many nutrients play a critical role in cellular development, oxygen transport, and other physiological functions within the body. Obesity is a known risk factor for several micronutrient deficiencies, and anemia, iron deficiency (ID), or a combination of both are common in patients with obesity before and after undergoing weight loss surgery.3 While there are several types of anemia, and nutrient deficiencies are not the primary cause for all, the majority of diagnosed anemias are linked to insufficient or deficient intake of key nutrients, including protein, iron, folate, and vitamins B6, B12, A, and E, as well as trace minerals selenium, copper, and zinc (Table 1).1,2 Iron deficiency, however, is the most prevalent micronutrient-related cause of anemia in pre- and postoperative bariatric surgery patients.4 This article reviews recent prevalence data of ID and iron deficiency anemia (IDA) in bariatric surgery patients and describes evidence-based pre- and postoperative ID and IDA screening, prevention, and treatment recommendations for bariatric patients undergoing weight loss surgery.
Iron Absorption, Excretion, and Storage
Overview. Iron is an essential mineral. Total body storage of iron for the typical adult man is 600 to 1,000mg, and for the typical adult woman, 200 to 300mg.5 Iron is stored primarily in the liver, spleen, red blood cells (RBCs), and bone marrow as ferritin and hemosiderin.5 Typically, iron is absorbed in the duodenum and upper jejunum, where it is transported for immediate use in the bone marrow or for storage. Compared to other minerals, iron balance is primarily regulated by absorption because the human body has no direct mechanism for iron excretion. Iron is lost minimally (approximately 1–2mg per day) through sloughing of epithelial cells or menstruation losses.6 It is estimated that 90 percent of daily iron needs in humans are obtained from endogenous sources, usually through the breakdown of RBCs.7 Thus, the bioavailability of dietary or supplemental iron is quite low and is highly dependent on the individual’s current iron status. When an individual has adequate iron stores, his or her body naturally absorbs less iron from dietary and/or supplemental sources; the opposite applies when an individual has low or no iron stores (i.e., his or her body will absorb more iron). Dietary sources of iron can be found in animal (heme) and plant (nonheme) sources, as well as in dietary supplements.
The estimated bioavailability of iron on the unaltered gastrointestinal (GI) tract of an individual who consumes an omnivore diet is 14 to 18 percent, and for those who primarily consume plant-based foods, 5 to 12 percent.7 These estimatations are based on individuals with no iron stores (serum ferritin <15mcg/dL), which suggests the maximum rate of iron absorption orally is less than 20 percent of intake. The World Health Organization (WHO) and Food and Agriculture Organization of the United Nations proposed iron bioavailability of 5, 10, 12, or 15 percent, with variances to account for individual iron status and dietary composition, such as enhancers (prebiotics [due to short-chain fatty acid fermentation], vitamin C and dietary muscle tissue [red meat, fish, poultry]) and inhibitors (phytates, polyphenols, calcium, and nonanimal tissue proteins [milk, egg, soybeans]) of iron absorption.7,8
A key hormonal regulator of iron homeostasis is hepcidin, a peptide produced in the liver and adipose tissue. In the presence of chronic inflammation and in individuals with obesity, hepcidin production is increased—leading to lower intestinal absorption of iron and less release of iron from storage cells.9–11
Iron absorption after bariatric surgery. The majority of bariatric surgery procedures will reult in altered iron absorption. For example, for patients who undergo Roux-en-Y gastric bypass (RYGB) procedures, altered iron absorption is caused by the reduced secretion of hydrochloric acid (HCl) due to bypassing of the gastric remnant and by bypassing the primary site of iron absorption, the duodenum.12 Once thought to have little to no effect on micronutrient absorption, the vertical sleeve gastrectomy (VSG) has also been found to have similar rates of long-term micronutrient deficiencies, compared to RYGB; it is speculated that the absorption of iron following this procedure is hindered due to resection of the greater curvature of the stomach and elimination of the majority of chief cells responsible for producing gastric acid, which might affect the liberation and dissolution of vitamins and essential trace metals, especially iron.12,13 Additionally, while bariatric surgery typically results in weight loss, the majority of patients remain in a chronic state of low-grade inflammation due to obesity, which also has been shown to decrease the absorption of iron.14
Postoperative absorption of iron has been studied in patients who have undergone RYGB or VSG, and investigators have found that both surgical procedures impact absorption negatively. In one study of patients who underwent RYGB surgery (N=36), iron absorption levels were collected preoperatively and then again at six, 12, and 18 months following surgery. At each postoperative follow-up time point, iron absorption was found to be 30 percent lower than the baseline absorption rate.15
In a study that compared heme and nonheme iron absorption in patients before and after undergoing RYGB (n=20) or VSG (n=20) procedures, researchers discovered that both surgical procedures resulted in a significant decrease in absorption of each type of iron.16 Surprisingly, they found no differences in surgical procedure absorption variance; thus, they pooled the results. For the complete study group, heme iron absorption averaged 23.9 percent (22.2–25.8%) prior to surgery, and 6.2 percent (5.3–7.1%) 12 months after surgery. Nonheme iron absorption averaged 11.1 percent (9.8–12.5%) prior to surgery, and 4.7 percent (3.1–5.5%) 12 months after surgery.16
A question remains regarding nutrient intestinal adaptation postoperatively in patients who have undergone weight loss surgery and whether there is a positive, negative, or neutral effect of various micronutrients absorption ability —this remains an interesting area for future study. However, current evidence suggests that the decrease in iron absorption in RYGB patients (up to 18 months postoperative) is seen immediately after surgery and remains unchanged.15 Additionally, long-term (up to 11 years postoperative RYGB and up to 5 years postoperative VSG) deficiency rates of ID and IDA are high, which suggests the possibility that the body does not begin to increase absorption of iron as the person progresses following surgery.12
ID and IDA. ID can develop any time iron homeostasis or iron metabolism is altered. Causes of ID include hemorrhage, insufficient iron absorption, insufficient iron intake, and/or increased hepcidin production. If ID is left untreated, impairment in the synthesis of hemoglobin can occur, which could result in anemia.6
Although ID is one of the most reported nutrient deficiencies before and after weight loss surgery, the problem may be underestimated or overlooked by healthcare professionals (HCPs) who treat postoperative weight-loss surgery patients due to its often late onset and possible parallel lack of adherence to follow-up appointments with the bariatric practice by the patient.17 Untreated ID can lead to significant morbidity and decreased quality of life in bariatric patients following weight loss surgery, with symptoms including fatigue, hair loss, brittle nails, angular cheilosis, sore tongue, and microcytic anemia.18 It can also negatively impact underlying cardiovascular or pulmonary disease in this patient population.13
ID and IDA before Bariatric Surgery
Prevalence. As new nutrition research data continues to emerge, it is important for clinicians to understand that, currently, 1) there is no universal definition for ID; and 2) iron status markers (and their reference ranges) vary from study to study, as does their sensitivity and specificity and duration of follow-up period within the study. Reported rates of deficiency must be interpreted with those factors in mind.
Generally accepted ranges for the prevalence of preoperative IDA and ID among bariatric surgery patients are 10 to 15 percent and 30 to 40 percent, respectively.19 In one study of 1,252 bariatric surgery patients, 28 percent were found to be iron deficient and 14 percent met the criteria for IDA prior to their surgery.19 In another study of 344 RYGB patients, less than 10 percent had ID or IDA prior to surgery; however, 12 months after surgery, 32 percent had ID and 16 percent were diagnosed with IDA.20
Screening recommendations. It is recommended that all bariatric patients are screened for nutritional deficiencies prior to weight loss surgery, and if an identified deficiency is found, that it be corrected prior to operating.1 However, clinical limitations exist when it comes to identifying and treating ID prior to surgery. One of the most challenging limitations includes the ability to identify whether treatment for IDA is effective because the average lifespan of an erythrocyte is approximately 120 days,21 and typical preoperative lab workup is conducted with a shorter duration to surgery than that. In the general surgical population, perioperative anemia has been linked to increased postoperative morbidity and mortality, as well as a decreased quality of life, whereas treatment of preoperative anemia has been shown to improve outcomes.22 There have been some research studies evaluating correction of micronutrient deficiencies prior to surgery and its positive effects on patient outcomes;23 however, with bariatric surgery, we have not fully elucidated the risk-to-benefit ratio of delaying surgery to correct a micronutrient deficiency.
A recent retrospective study evaluated whether correcting micronutrient deficiencies prior to VSG could help prevent deficiencies following surgery. Two patient groups were compared, those who had micronutrient deficiencies repleted prior to surgery (Group A, n=42), and those who entered surgery with a known micronutrient deficiency (Group B, n=38). Both groups were provided with the same dietary instruction (including supplement recommendations), and serum markers were evaluated preoperatively and at Month 3 and Month 12 following surgery. Follow-up at Month 3 and Month 12 revealed that Group A continued to have no identified micronutrient deficiencies, whereas Group B continued to be deficient in one or more micronutrient at each time-point despite routine supplementation.24 This study adds to the data that support the recommendation of repletion of micronutrient deficiencies prior to weight loss surgery, but whether a micronutrient deficiency should absolutely be resolved before surgery is done remains a question for most clinicians, and individual patient needs must be considered. Additionally, many patients do not receive a full nutrition workup prior to surgery; an undetected preoperative deficiency might place a patient at greater risk for an exacerbated postoperative deficiency that is more difficult to correct. The 2013 American Association of Clinical Endocrinologists (AACE)/The Obesity Society (TOS)/American Society for Metabolic and Bariatric Surgery (ASMBS) guidelines state that if a patient is unable to receive preoperative nutrition screening, he or she can start on preventative postoperative oral micronutrient supplementation in lieu of receiving a full nutrition lab work-up. Although this is not ideal, it might be a practical clinical consideration for some patients. See Table 2 for diagnostic considerations and Table 3 for physical signs and symptoms of deficiency.
ID and IDA after Bariatric Surgery
Prevalence. Prevalence of IDA after VSG and RYGB are reported to be as high as 50 and 65 percent, respectively.12 This means a significant portion of people who have bariatric surgery will develop ID or IDA. While it was once thought to be primarily a concern for RYGB patients, recent data suggest that VSG patients have comparable ID and IDA rates. In a recent evidence update, long-term rates of ID in VSG and RYGB were 15 to 45 percent and 25 to 50 percent, respectively.13
One longitudinal study (N=959; 84.9% female), which assessed the 10-year outcome of ID incidence after RYGB, found that 53 percent of the patients had ID at some point in the postoperative period and that 22.7 percent met the criteria for IDA. This led the researchers to conclude that female bariatric surgery patients should be counseled that there is a 50 percent chance they will become iron deficient at some point following RYGB surgery.25
Screening recommendations. Because there is not a concrete definition of ID, nor is there one serum marker that is an absolute indicator of iron deficiency, a combination of markers is often used and recommended for screening of potential deficiency. For example, factors within the body, including inflammation, aging, and infection, affect certain ferritin levels, which is often the first serum marker assessed for ID. Serum iron is also not an absolute marker because it is influenced by diurnal variations and additional external factors.26
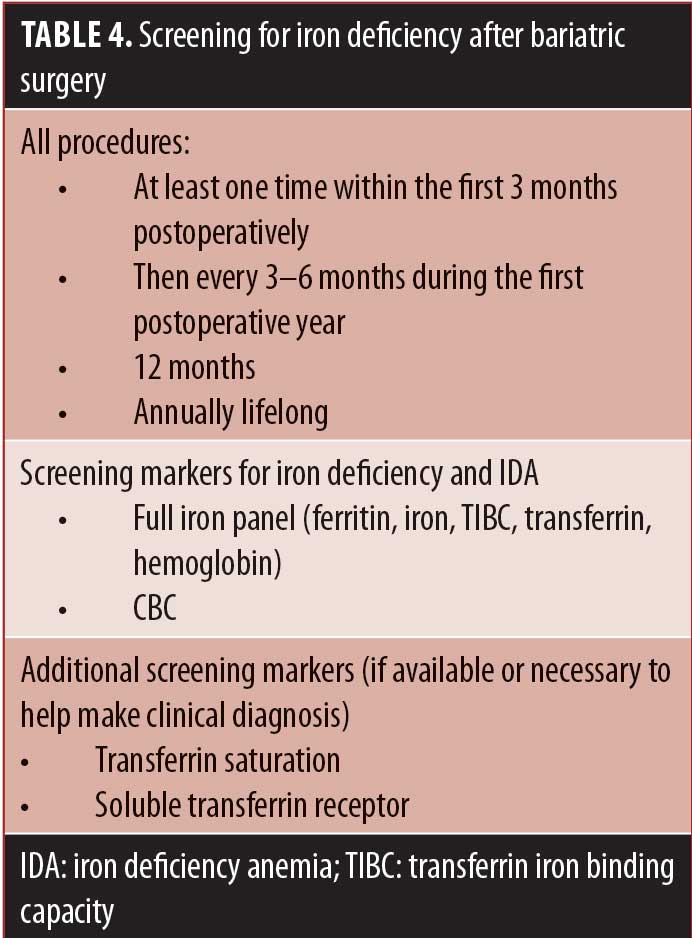
Ferritin is an acute-phase reactant, and false normal levels of ferritin could be a concern if ferritin is the only marker being used to assess iron status. Hemoglobin and hematocrit changes are less sensitive in identifying early anemia and changes often reflect late IDA—for these reasons a full iron panel is recommended at all screening points before and after weight loss surgery. Soluble transferrin receptor level is an additional serum marker that can be added, which is less impacted by inflammation and reflects the cellular need for iron.27 Ferritin is, however, a specific and early indicator of ID, in the absence of inflammation or infection, and low ferritin with normal iron levels indicates a reduced body iron capacity.28 To assess for inflammation, a standard C-reactive protein (CRP) can be included. If CRP levels are above 3, with a normal ferritin level (as high as 100), a patient should be suspected to have lower than expected oral iron absorption.29 A 2015 meta-analyses reviewed 5,909 bariatric patients who had RYGB surgery and found low ferritin levels increased twofold at the six-month postoperative follow-up compared to preoperative levels.30 However, the investigators found no change in serum iron concentrations and suggested that low ferritin levels with stable serum iron indicates reduced body iron capacity.
See Table 4 for a summary of postoperative ID and IDA screening recommendations.
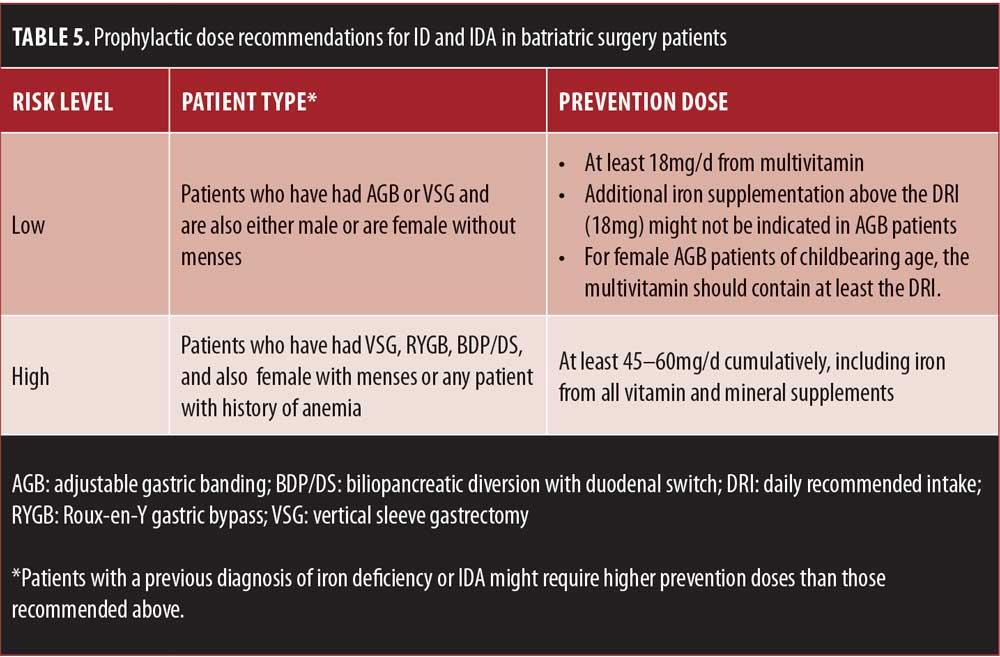
Prophylactic supplement recommendations. Prevention of ID and IDA is of utmost importance as ID and IDA treatment can be a challenge to orally replete. If ID is detected early, it is often easy to correct with oral micronutrient supplementation; however, if the severity of the deficiency is high, repletion with oral supplementation will likely not be adequate. Current US prevention guidelines for bariatric surgery patients vary according to sex, menstruation status, and procedure type.28,31
In one study evaluating bariatric patients following RYGB surgery (N=36, average length of postoperative follow-up time was 6.8 years), the investigators aimed to identify the influence of diet and supplements on the iron status. They found that ID was present in 57 percent of nonusers of iron supplements and in 15 percent of iron supplement users, and that high intakes of dietary heme iron and vitamin C and adherence to prophylactic iron supplementation were associated with improved levels of iron status biomarkers.32
See Table 5 for a summary of prophylactic dose recommendations.
Postoperative repletion recommendations. Risks of postoperative ID and IDA should be considered. In an analysis that aimed to compare the medical costs and complications associated with anemia after bariatric surgery, it was discovered that of the 24,344 patients analyzed, 11.6 percent received an IDA diagnosis within two years of surgery.31 Investigators found that the overall complication rate was 40.4 percent for the group with IDA, with nearly twice the number of hospitalizations, and 27.7 percent for the group without IDA. For those who were hospitalized, the IDA group stayed an average three day longer in the hospital than the non-IDA group (8.3 vs. 5.2 days). Total medical resource utilization (MRU) two-year private payer reimbursement cost (adjusted at 2012 US dollars) was almost double in the IDA group ($37,882 vs. $19,253). Another study showed a similar increase in medical costs for patients with an IDA diagnosis (almost twice the amount of US dollars spent), compared to non-IDA patients with the same comorbid conditions.34
If an identified deficiency is found, repletion via oral supplements is often the first line of defense. If discovered and properly treated early enough, studies have shown that oral repletion postoperatively is possible. In one study of bariatric patients who underwent RYGB (N=796), 17.4 percent were diagnosed with postoperative IDA. Median duration from surgery to diagnosis was 13 months, and oral iron led to an 81-percent overall positive response. Of that cohort, 13.5 percent of patients needed intravenous (IV) iron due to intolerance of oral supplementation.35 If the degree of ID or IDA is severe enough upon first discovery, or if the patient is unwilling or unable to follow the recommended dosing strategy, then parental or intravenous (IV) iron infusions might be necessary.
Repletion guidelines range, requiring clinical judgment based on individual patient need when devising a treatment plan. The repletion range of daily elemental iron suggested by recent guidelines for bariatric surgery patients are, for all procedures and all patients 100 to 300mg of elemental iron daily 2 to 3 times daily.1
Many factors should be considered when recommending the dose of oral iron for repletion of a deficiency, including patient tolerance of oral iron, severity of ID or IDA, and patient willingness to take the recommended dose. It is recommended that bariatric surgery patients take their iron in divided doses, away from foods high in phytates, polyphenols, and calcium supplements, to maximize iron absorption.1 The most common complaint from bariatric patients when taking high doses of oral iron supplementation is gastric distress, including nausea and constipation. Depending on individual tolerance, titrating up from a smaller amount of iron to the recommended daily dose might be warranted. Changing forms of supplementation might also help due to variance in individual tolerance to different iron salts (e.g., fumarate, gluconate, sulfate).36
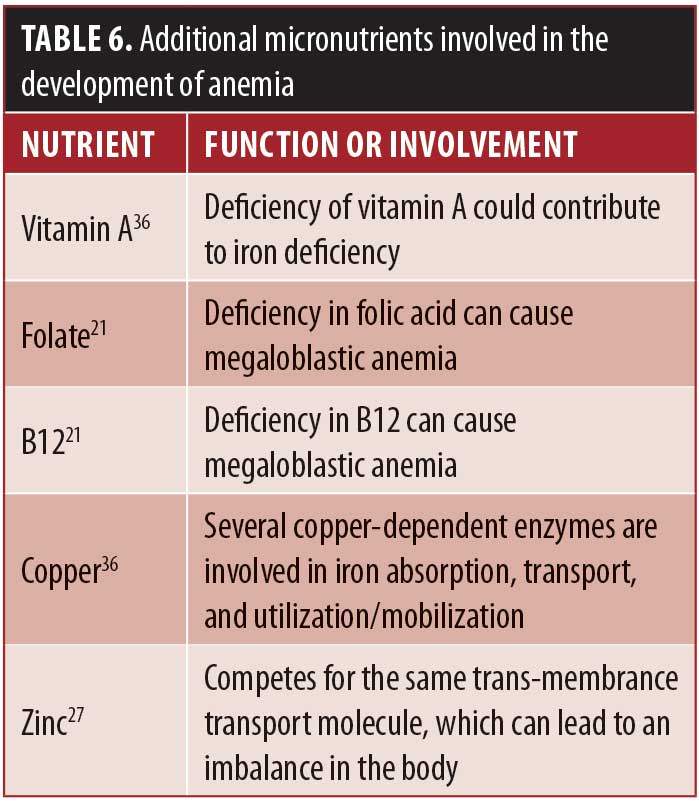
Trends in serum markers should be reassessed in 4-to-8-week intervals until iron status returns to normal.36 A reticulocyte index can be ordered after initiation of oral iron therapy to see if a patient is responding to treatment. Reticulocytes are immature RBCs and can be expected to increase as early as 7 to 10 days after beginning high-dose oral iron therapy.36 If a patient does not respond to initial oral therapy, the oral dose should be increased or the patient referred to parenteral or IV iron repletion. In one retrospective study of patients (N=42) with postoperative IDA who were nonresponsive to oral supplementation (either ferrous sulfate 325mg [elemental iron 65mg] 3 to 4 times daily or ferrous fumarate 324mg [elemental iron 106mg] twice daily), IV iron was administered with varying frequency from once every four months to once every 11 months to correct their anemia.37 Some case reports have shown rapid and complete improvement in hemoglobin levels and other iron parameters after IV iron administration,19 while others have shown persistent anemia with a need for repeat IV iron supplementation.38 It is important to note that in addition to the general precautions regarding IV infusions, iron administration requires test dosing due to its association with rare anaphylaxis.39 Additionally, if anemia is persistent, assessing for additional micronutrient deficiencies is indicated. Table 6 lists functions of additional micronutrients involved in iron metabolism and anemias. In addition to other micronutrient deficiencies, medical conditions have been shown to influence iron status as well. Eradicating an active Helicobacter pylori (H. pylori) infection, for example, has been shown to improve response to iron supplementation.40
Conclusion
Although there are many positive health outcomes after bariatric surgery, it is important to remember that obesity is a chronic disease that presents with risk factors associated with severe micronutrient deficiencies. Hematological disorders after surgery, due in part to micronutrient deficiencies, can decrease the patient’s ability to achieve optimal improvements in quality of life and health following bariatric surgery. Screening for micronutrient deficiencies before bariatric surgery and life-long screening after bariatric surgery, along with patient education on prevention doses of micronutrients, immediate recognition of deficiencies, and proper treatment and follow-up are key helping our bariatric patients optimize their weight loss surgery outcomes.
References
- Parrott J, Frank L, Rabena R, et al. ASMBS Integrated Health Nutritional Guidelines for The Surgical Weight Loss Patient—2016 update: micronutrients. Surg Obes Relat Dis. 2016;12:955–959.
- Gletsu-Miller N, Wright BN. Mineral malnutrition following bariatric surgery. Adv Nutr. 2013;4:506–517.
- Moize V, Deulofeu R, Torres F, et al. Nutritional intake and prevalence of nutritional deficiencies prior to surgery in a Spanish morbidly obese population. Obes Surg. 2011;21:1382–1388.
- Gribsholt S, Nielsen J, Melen C, et al. Work-up and treatment of iron deficiency anemia after bariatric surgery with gastric bypass. Ugeskr Laeger. 2014;24;176.
- Saito H. Metabolism of iron stores. Nagoya J Med Sci. 2014;76(3-4):235–254.
- Andrews NC. Disorders of iron metabolism. N Engl J Med. 1999;11:139–146.
- Hurrell R, Egli I. Iron bioavailability and dietary reference values. Am J Clin Nutr. 2010;91(Suppl):1461S–1467S.
- WHO/FAO. Vitamin and mineral requirements in human nutrition. 2nd ed. Geneva, Switzerland:World Health Organization and Food and Agriculture Organization of the United Nations, 2004.
- Ganz T, Nemeth E. Iron import. IV. Hepcidin and regulation of body iron metabolism. Am J Physiol Gastrointest Liver Physiol. 2006;290:G199–203.
- Yanoff LB, Menzie CM, Denkinger B, et al. Inflammation and iron deficiency in the hypoferremia of obesity. Int J Obes (Lond). 2007;31:1412–1419.
- Benotti P, Wood C, Still C. Iron nutrition and metabolic surgery: the next quality improvement challenge. Bariatric Times. 2019;16(3):8–11.
- Steenackers N, Van der Schueren B, Mertens A, et al. Iron deficiency after bariatric surgery: what is the real problem? Proc Nutr Soc. 2018;77:445–455.
- Via M, Mechanick J. Nutritional and micronutrient care of bariatric surgery patients: current evidence update. Curr Obes Rep. 2017;6:286–296.
- Benotti P, Wood G, Still C, et al. Metabolic surgery and iron homeostasis. Obes Rev. 2018;1–9.
- Ruz M, Carrasco F, Rojas P. Iron absorption and iron status are reduced after Roux-en-Y gastric bypass. Am J Clin Nutr. 2009;90:527–532.
- Ruz M, Carrasco F, Rojas P, et al. Heme- and nonheme-iron absorption and iron status 12 mo after sleeve gastrectomy and Roux-en-Y gastric bypass in morbidly obese women. Am J Clin Nutr. 2012;96:810–817.
- Fox W, Borgert A, Rasmussen C, et al. Long-term micronutrient surveillance after gastric bypass surgery in an integrated healthcare system. Surg Obes Relat Dis. 2019;15(3):389–395.
- Han O. Molecular mechanism of intestinal iron absorption. Metallomics. 2011;3(2):103–109.
- Munoz M, Botella-Romero F, Gomez-Ramirez S, et al. Iron deficiency and anaemia in bariatric surgical patients: causes, diagnosis and proper management. Nutricion Hospitalaria. 2009;24:640–654.
- Aarts EO, van Wageningen B, Janssen IM, et al. Prevalence of anemia and related deficiencies in the first year following laparoscopic gastric bypass for morbid obesity. J Obes. 2012;2012:193705.
- Litchford M. Laboratory Assessment of Nutritional Status: Bridging Theory and Practice. Greensboro, NC: CASE Software, 2017.
- Shander A, Knight K, Thurer R, et al. Prevalence and outcomes of anemia in surgery: a systematic review of the literature. Am J Med. 2004;116:58S–69S.
- Nelson M, Bolduc L, Toder M, et al. Correction of preoperative vitamin D deficiency after Roux-en-Y gastric bypass surgery. Surg Obes Relat Dis. 2007;3(4):434–437.
- Schiavo L, Pilone V, Rossetti G, et al. Correcting micronutrient deficiencies before sleeve gastrectomy may be useful in preventing early postoperative micronutrient deficiencies. Int J Vitam Nutr Res. 2019;89(1–2):22–28.
- Obinwanne K, Fredrickson K, Mathiason M, et al. Incidence, treatment, and outcomes of iron deficiency after laparoscopic roux-en-y gastric bypass: a 10-year analysis. J Am Coll Surg. 2014;218:246–252.
- Auerbach M & Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2015;91:31–38.
- Parrott J, Frank L, Rabena R, et al. ASMBS Integrated Health Nutritional Guidelines for The Surgical Weight Loss Patient—2016 update: micronutrients. Surg Obes Relat Dis. 2017;13(5):727–741.
- Goddard AF, James MW, McIntyre AS, et al. Guidelines for the management of iron deficiency anaemia. Gut. 2011;60:1309–1316.
- Careaga M, Moize V, Flores L, et al. Inflammation and iron status in bariatric surgery candidates. Surg Obes Rel Dis. 2015;11:906–911.
- Weng TC, Chang CH, Dong YH, et al. Anaemia and related nutrient deficiencies after Roux-en-Y gastric bypass surgery: a systematic review and meta-analysis. BMJ Open. 2015;5:e006964.
- Mechanick, Jeffrey I, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient—2013 update: cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Obesity (Silver Spring). 2013;21 Suppl (0 1): S1–27.
- Mischler R, Armah S, Wright B, et al. Influence of diet and supplements on iron status after gastric bypass surgery. Surg Obes Relat Dis. 2016;12:651–658.
- Knight T, D’Sylva L, Moore B, et al. Burden of iron deficiency anemia in a bariatric surgery population in the United States. J Manag Care Spec Pharm. 2015;21(10):946–954.
- Nissenson A, Wade S, Goodnough T, et al. Economic burden of anemia in an insured population. J Manag Care Pharm. 2005;11(7):565–574.
- Kyoung Ha K, Min-Young L, Jo Kim H, et al. Prevalence of anemia and treatment outcomes after bariatric surgery —a retrospective Korean study. Blood. 2015;126:4562.
- Jacques J. Micronutrition for the Weight Loss Surgery Patient. Edgemont, PA: Matrix Medical Communications, 2006.
- Varma S, Baz W, Badine E, et al. Need for parenteral iron therapy after bariatric surgery. Surg Obes Relat Dis. 2008;4:715–719.
- DeFilipp Z, Lister J, Gagne D, et al. Intravenous iron replacement for persistent iron deficiency anemia after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2013;9:129–132.
- Silverstein S, Rodgers G. Parenteral iron therapy options. Am J Hematol. 2004;76:74–78.
- Hershko C, Hoffbrand AV, Keret D, et al. Role of autoimmune gastritis, Helicobacter pylor and celiac disease in refractory or unexplained iron deficiency anemia. Haematologica. 2005;90(5):585–595.
Category: Past Articles, Review







