Raising the Standard: Adapting Advanced Hemodynamic Monitoring to Optimize Surgical Outcomes in Bariatric Surgery

by Sabatino Leffe, DO; Don Decrosta, MD; Dominick Gadaleta, MD, FACS, FASMBS; and anthony T. Petrick, MD, FACS, FASMBS
Dr. Leffe is Vice Chair, Department of Anesthesiology, South Shore University Hospital; Eastern Region Operations Director, Northwell Anesthesiology, Northwell Health in Manhasset, New York; and Assistant Professor of Anesthesiology, Zucker School of Medicine at Hofstra in Hempstead, New York. Dr. Decrosta is Chair, Department of Anesthesiology, South Shore University Hospital; Northwell Anesthesiology, Northwell Health in Manhasset, New York; and Assistant Professor of Anesthesiology, Zucker School of Medicine at Hofstra in Hempstead, New York. Dr. Gadaleta is Vice Chair, Department of Surgery, Northwell Health; Chief, Division of General Surgery, North Shore University Hospital in Manhasset, New York; and Professor of Surgery, Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. Dr. Petrick is Chief Quality Officer, Geisinger Clinic; Director of Bariatric and Foregut Surgery, Geisinger Health System in Danville, Pennsylvania.
Funding: No funding was provided for this article.
Disclosures: The authors report no conflicts of interest relevant to the content of this article.
Bariatric Times. 2023;20(3–4):22–24.
Rapid advances in bariatric surgical techniques and the development of minimally invasive surgical platforms have made bariatric surgery the most durable and effective treatment for obesity and its associated comorbidities. The positive risk-benefit ratio has been demonstrated by multiple studies, with one study showing 90-day mortality to be as low as 0.35 percent.1 Anastomotic leak, a major complication of bariatric surgery, can occur in 1 to 5 percent of gastric bypass surgeries and 1 to 3 percent of sleeve gastrectomies; once a leak occurs, mortality can be as high as 16 percent.2
The focus on preventing anastomotic leaks has traditionally been on nutrition; optimization of preoperative comorbid disease states, such as obstructive sleep apnea (OSA) and diabetes; determining surgical best practices; and checking for leaks intraoperatively. Microcirculatory blood flow has been demonstrated to be critical in anastomotic healing. Commonly employed and often poorly defined intraoperative goals include maintaining euvolemia and normotension and avoiding acute anemia through blood loss to maintain tissue oxygenation at the level of anastomosis. Recent Enhanced Recovery After Surgery (ERAS) Society guidelines for bariatric surgery strongly recommend avoiding restrictive or liberal perioperative fluid management in favor of individual, goal-directed therapy.3
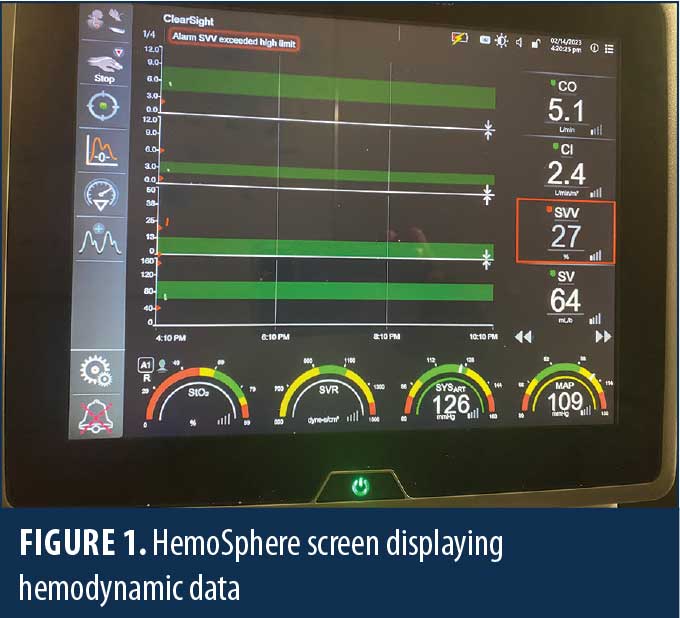
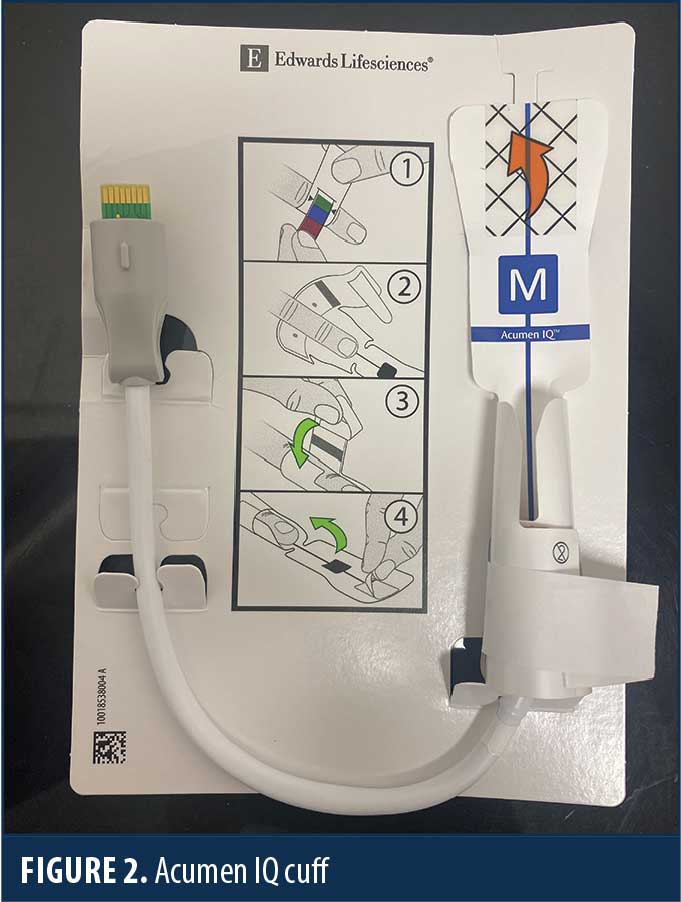
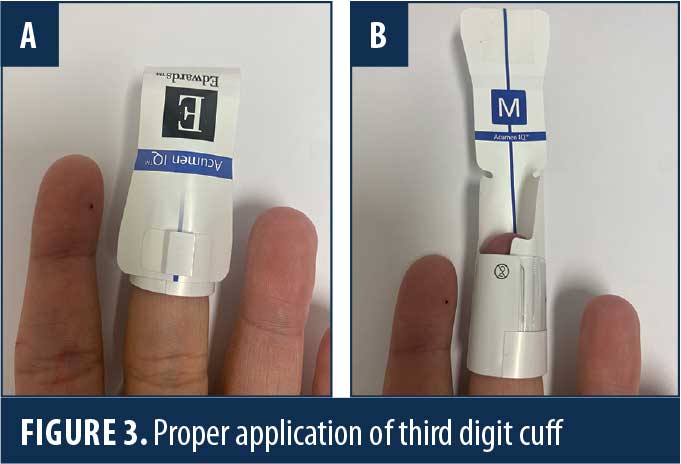
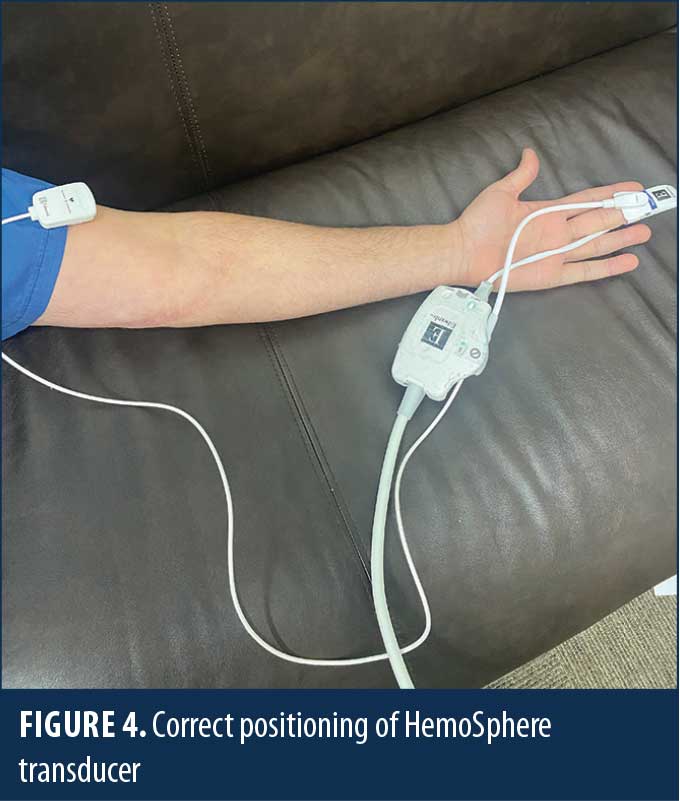
The HemoSphere (Edwards Lifesciences; Irvine, CA, United States [US]) is an advanced hemodynamic monitoring system that harnesses predictive algorithms and sophisticated software to provide advanced pressure, flow, and oximetry insights on a single monitor (Figure 1). The data indices measured more clearly define specific intraoperative hemodynamic derangements and help guide more precise and timely therapy. This technology can be adapted to a traditional invasive intra-arterial catheter or a third digit finger pressure cuff (Acumen IQ cuff; Edwards Lifesciences; Irvine, CA, US), which provides a noninvasive means to continuously monitor hemodynamics (Figures 2–4). Finger cuffs come in three different sizes to accommodate patient variability and ensure accuracy.
Recent publications confirm the accuracy of ClearSight (Edwards Lifesciences; Irvine, CA, US), with one study demonstrating a strong correlation of the mean arterial pressure and diastolic blood pressure when comparing ClearSight finger cuff and an intra-arterial catheter.4–6 The correlation was found to be significantly better than traditional upper extremity noninvasive oscillometric measurements, which can underestimate the severity of intraoperative hypotension.
Acumen Hypotensive Predicative Index (HPI) is a proprietary software feature used with the Acumen IQ cuff (Edwards Lifesciences; Irvine, CA, US) that provides real-time data, including mean arterial pressure (MAP), cardiac index (CI), systemic vascular resistance (SVR), and stroke volume (SV). The HPI is measured continuously and predicts hypotension before it occurs. This enables the anesthesiologist to intervene and treat hypotension before traditional hemodynamic parameters, such as blood pressure and urine output, deteriorate.
More advanced hemodynamic data can also guide therapeutic interventions more precisely, including volume replacement for preload deficits, vasopressor therapy for afterload/SVR deficits, or inotropic support for decreased cardiac contractility and cardiac output. Some studies have demonstrated a reduction in the duration of hypotensive episodes compared with historical controls.7,8 Hypotension, even for brief periods, has been demonstrated to have deleterious effects on kidney function, readmission rates, and mortality.9 Avoiding hypotension should be a goal in bariatric surgery to promote optimal anastomotic microcirculation and maximize tissue viability.
Acumen Assisted Fluid Management (AFM) is software that tracks continuous blood pressure and cardiac output and provides a quantitative measure of SV variation (SVV), which correlates strongly with the degree of hypovolemia. It can guide the perioperative administration of intravenous (IV) fluids, presumably preventing overhydration and potential tissue edema, which might adversely alter microcirculation.
SVV is a naturally occurring phenomenon in which the arterial pulse pressure falls during inspiration and rises during expiration due to changes in intrathoracic pressure, secondary to negative pressure ventilation (spontaneously breathing). Variations over 10mmHg have been referred to as pulsus paradoxus. The normal range of variation in spontaneously breathing patients has been reported between 5 to 10mmHg.10
Reverse pulsus paradoxus occurs with controlled mechanical ventilation as intrathoracic pressures dynamics are reversed. Arterial pressure rises during inspiration and falls during expiration, secondary to positive pressure ventilation. Traditionally, SVV is calculated by taking the mean of SVmax–SVmin/SV over a respiratory cycle or other period of time.
SVV and its comparable measurement, pulse pressure variation (PPV), are not indicators of actual preload but of relative preload responsiveness. According to Edwards,10 SVV has a very high sensitivity and specificity compared to traditional indicators of volume status, such as heart rate, mean arterial pressure, central venous pressure, peripheral arterial disease, pulmonary artery occlusion pressure, and their ability to determine fluid responsiveness. This allows for greater precision in predicting fluid responsiveness against a specified infused volume.
Normal SVV values are less than 10 to 15 percent on controlled mechanical ventilation. In our institution, in accordance with Edwards, we target a goal SVV of less than 13 percent. Unfortunately, this applies only to mechanically ventilated patients and can be rendered inaccurate with arrythmias (e.g., atrial fibrillation), decreased vascular tone, or high positive end-expiratory pressure. In this setting, we utilize the SVV as a surrogate measure of volume deficit.
Judicious, low-volume intermittent boluses of IV fluid can be administered and SVV continuously monitored to prevent unnecessary large volumes of crystalloid, while preventing underhydration. When used within its limitations, SVV is a sensitive tool that can be used to guide the appropriate management of the patient’s preload to achieve optimal oxygen delivery and optimize the role of fluids in improving hemodynamics.
Our department has also instituted the integration of data provided by the HemoSphere in conjunction with other modern anesthetic patient monitoring devices, in particular the bispectral index (BIS) monitor. With a BIS target of 40 to 60, we utilize a comprehensive anesthetic protocol of total IV anesthesia (TIVA), including propofol, ketamine, and lidocaine infusions, and carefully titrated, nondepolarizing neuromuscular blockade with preoperative transversus abdominus plane (TAP) blocks, using long-acting liposomal bupivacaine. This provides excellent surgical conditions, stable hemodynamics, and excellent postoperative analgesia for most patients. More importantly, this avoids the need for significant perioperative doses of opioids. Post-bariatric surgery patients are at particularly high risk for opioid dependence, with up to 14.2 percent of opioid-naïve patients reporting opioid use at seven years following their operation, as demonstrated in the Longitudinal Assessment of Bariatric Surgery study.11 We find our patients seldom require postoperative IV boluses of opioid rescue medication.
Equally important, by carefully titrating our anesthetic technique to a narrow BIS score range, any incidence of hypotension or a decrease in SVV is theoretically less likely to occur due to excessive administration of anesthetic agents and resultant vasodilation.
Advanced hemodynamic monitoring has been used in critical care and trauma settings through previous technology (Flo-trac; Edwards Lifesciences; Irvine, CA, US). The HemoSphere provides accurate, widely applicable, noninvasive technology that makes use of an advanced hemodynamic monitoring routine. It can guide future studies, which will help define optimal hemodynamic goals in bariatric surgery and hopefully improve surgical outcomes.
References
- Pories WJ. Bariatric surgery: risks and rewards. J Clin Endocrinol Metab. 2008;93(11 Suppl 1):S89–S96.
- Pooli A, Phillips E. Prevention of anastomatic leaks in bariatric surgery. Bariatric Times. 2010;7(3):8–13.
- Stenberg E, Dos Reis Falcão LF, O’Kane M, et al. Guidelines for perioperative care in bariatric surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations: a 2021 update. World J Surg. 2022;46(4):729–751.
- Frassanito L, Giuri PP, Vassalli F, et al. Hypotension Prediction Index with non-invasive continuous arterial pressure waveforms (ClearSight): clinical performance in gynaecologic oncologic surgery. J Clin Monit Comput. 2022;36(5):1325–1332.
- Rogge DE, Niklaus JY, Haas SA, et al. Continuous noninvasive arterial pressure monitoring in obese patients during bariatric surgery: an evaluation of the vascular unloading technique (Clearsight system). Anesth Analg. 2019;128(3):477–483
- Schumann R, Meidert AS, Bonney I, et al. Intraoperative blood pressure monitoring in obese patients. Anesthesiology. 2021; 134(2):179–188.
- Schneck E, Schulte D, Habig L, et al. Hypotension Prediction Index based protocolized haemodynamic management reduces the incidence and duration of intraoperative hypotension in primary total hip arthroplasty: a single centre feasibility randomised blinded prospective interventional trial. J Clin Monit Comput. 2020;34:1149–1158
- Davies SJ, Vistisen ST, Jian Z, et al. Ability of an arterial waveform analysis-derived Hypotension Prediction Index to predict future hypotensive events in surgical patients. Anesth Analg. 2020;130(2):352–359.
- Walsh M, Devereaux PJ, Garg AX, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology. 2013;119(3):507–515.
- Frazier J. Clinical Marketing, Edwards Lifesciences “Stroke Volume Variation: Can we use fluids to Improve Hemnodynamics?”
- King WC, Chen JY, Belle SH, et al. Use of prescribed opioids before and after bariatric surgery: prospective evidence from a US multicenter cohort study. Surg Obes Relat Dis. 2017;13(8):1337–1346.
Category: Past Articles, Raising the Standard



