Small Intestinal Bacterial Overgrowth in Modern Bariatric Surgery
 by Diana Miao, BA
by Diana Miao, BA
Diana Miao, BA is with the Beth Israel Deaconess Medical Center and Harvard Medical School.
Funding: No funding was provided.
Disclosures: The author reports no conflict of interest related to the content of this article.
Abstract: Small intestinal bacterial overgrowth (SIBO) is a rare complication of Roux-en- Y gastric bypass, but was relatively common following jejunoileal bypass (JIB) and can lead to severe malabsorption and serious autoimmune systemic disease. Prompt recognition of this condition can guide appropriate antibiotic therapy, thus preventing complications from chronic total protein malnutrition or vitamin and micronutrient deficiencies. Furthermore, the gut microbiome undergoes substantial changes in all patients who have undergone gastric bypass surgeries and might have a prominent role in regulating post-operative weight loss. Further research at the intersection of bariatric surgery and the gut microbiome can increase understanding of the mechanisms of weight loss following malabsorptive surgical procedures, as well as enhance clinical recognition of rare complications like SIBO that can lead to adverse patient outcomes.
Keywords: Roux-en-Y gastric bypass, jejunoileal bypass, microbiome, SIBO
Bariatric Times. 2017;14(11):16–19.
Introduction
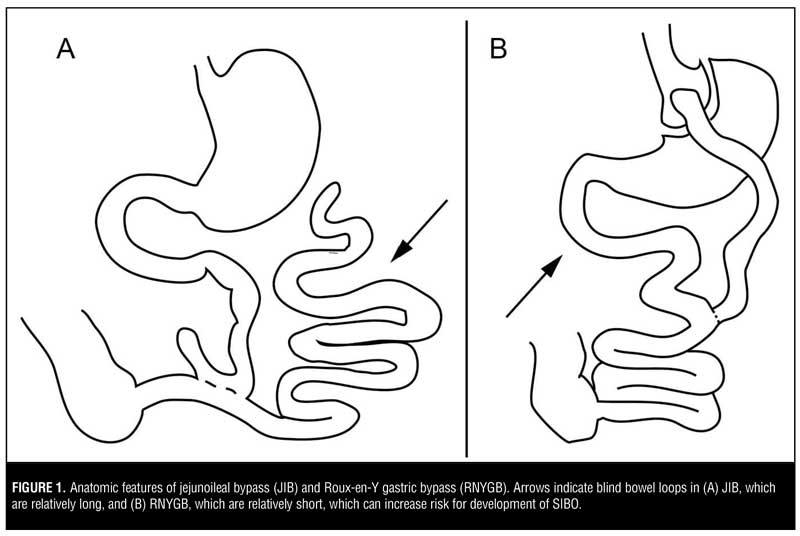
Bariatric surgery has been shown to promote weight loss and slow or reverse progression of comorbid conditions, such as diabetes, obstructive sleep apnea, hyperlipidemia, and hypertension, in adults and adolescents.[1,2] In comparison to restrictive bariatric procedures (gastric sleeve, gastric balloon, gastric band), the malabsorptive procedures [Roux-en-Y gastric bypass (RNYGB), jejunoileal bypass, biliopancreatic switch] are associated with greater weight loss but can also lead to idiosyncratic complications, as the creation of blind loops of the small bowel can be sites for small intestinal bacterial overgrowth (SIBO) (Figure 1).
SIBO Overview
The small intestine normally harbors fewer than 103 bacteria/mL, and SIBO is defined as small intestinal bacterial populations exceeding 105–106 bacterial organisms/mL. SIBO develops in a variety of clinical conditions involving gastric acid suppression, disordered gastrointestinal motility, or structural abnormalities of the gastrointestinal tract, all of which are permissive for bacterial proliferation.[3] Symptoms range from diarrhea and bloating to weight loss and vitamin deficiencies, depending on the type of colonizing microorganism.
No widely accepted gold standard for diagnosis of SIBO exists.[4] Bacterial culture of small bowel luminal content is the most direct test, but this method is time-consuming, cumbersome, and might underestimate bacterial populations that are difficult to culture. Clinically, measuring levels of hydrogen gas—presumably produced by anaerobic bacteria abnormally colonizing the small bowel—that are exhaled after a fixed dose of oral glucose or lactulose is the most common test for SIBO. Observing elevated breath hydrogen levels compared to fasting, or seeing two peaks in hydrogen production after the oral carbohydrate load, are considered a positive result. Diagnosing SIBO in bariatric patients is especially challenging because symptoms mimic those of bariatric surgery in general, and hydrogen breath tests can be misleading due to anatomic alterations. However, accurate identification is important in order to properly treat patients.
Accurate Diagnosis of SIBO Following Bariatric Surgery
Gastric bypass surgery results in faster small bowel transit time, such that the hydrogen breath test can be misleading if measured at a standard time after oral carbohydrate load. This is because the carbohydrate bolus might have already reached the colon, where anaerobic metabolism is normal. In one study, 19 patients with RNYGB experiencing diarrhea, bloating, flatulence, and abdominal pain underwent simultaneous lactulose breath testing to assess presence of bacterial growth and upper GI series with small bowel follow-through to assess orocecal transit time. Nine patients had positive lactulose breath test (>20ppm rise in hydrogen above baseline within 90 minutes), of whom six had orocecal transit time preceding the time to rise in exhaled hydrogen, suggesting that the measured hydrogen was produced by normal colonic flora, not bacteria abnormally colonizing the small intestine. All nine patients received oral antibiotics for presumed SIBO, but only the three with orocecal transit times exceeding the time of rise of breath hydrogen responded to treatment[5]. Thus, accurate diagnosis and appropriate treatment of SIBO in patients with nonspecific and mild symptoms might require specialized protocols in post-gastric-bypass patients. To describe more drastic presentations of SIBO, referring to historical experience with jejunoileal bypass (JIB) is illuminating.
JIB and SIBO
JIB was an early form of bariatric surgery that was popular in the 1960s and 70s and has since largely been replaced by gastric bypass due to the high rate of complications, including diarrhea, vitamin deficiencies, and asthenia.[6] The exact mechanisms of all these complications remain unclear, but bacterial colonization of the bypassed small bowel might be one of the culprits. For example, experimental studies in rats have found that 90-percent small bowel resection causes 15-percent reduction in weight, while 90-percent bypass leads to twice as much weight loss. This effect can be nearly eliminated by treatment with metronidazole but not cephalexin, suggesting that bacterial overgrowth in the excluded blind loop of the small bowel might contribute to post-surgical malabsorption.[7] Besides excess weight loss, other complications of JIB thought to be related to SIBO include bypass enteritis, hypovitaminosis B12, proctitis, encephalopathy, liver disease, polyarthritis, nephrolithiasis, cholelithiasis, and dermatitis, based on the detection of bacterial antigen/antibody complexes involved in these conditions or the observation that antibiotic treatment was clinically effective in reducing symptom severity.[8]
One of the most well-described SIBO-associated complication is the bowel-associated dermatosis-arthritis syndrome (BADAS), which occurs in about 6.5 percent of patients following JIB or up to one third of patients undergoing jejunocolonic bypass.[9] This condition manifests as a maculopapular rash that usually affects the trunk and arms, flu-like symptoms, polyarthralgias, and myalgias developing three months to five years after surgery. The pathogenesis of this disorder involves bacterial peptidoglycans,[10] which lead to alternative pathway complement activation, production of circulating immune complexes, IgM and IgG deposition in dermal vessels and the dermoepidermal junction, and neutrophilic extravasation into the skin.[11] Cryoglobulinemia is also common, and immunoglobulins to Escherichia coli (E coli) and Bacteroides fragilis (B fragilis) have been detected in some patients.[12,13] Interestingly, BADAS has also been observed in non-bypass surgery patients with chronic bowel inflammation, including inflammatory colitis and diverticulitis,[11] further supporting this syndrome’s mechanistic relationship to bacterial overgrowth. Researchers have also reported the occurance of BADAS in other surgical procedures that create a blind bowel loop, including biliopancreatic diversion,[14] RNYGB,[15] partial gastrectomy with Roux-en-Y jejunostomy,16 and Billroth II gastrectomy.[17] Thus, SIBO-associated complications following JIB are not just a topic of historical interest, but remain clinically relevant to understanding complications of infectious or immune-mediated gastrointestinal diseases and of surgical procedures that create a blind bowel loop.
Roux-en-Y Gastric Bypass and SIBO
As JIB has been phased out of surgical practice, SIBO-related complications of bariatric surgery have received less clinical attention. Rates of development of post-surgical SIBO have dropped, and symptoms of SIBO resemble those observed following RNYGB and biliopancreatic diversion with duodenal switch procedures themselves, including weight loss, steatorrhea, abdominal discomfort and bloating, vitamin deficiencies (especially A, D, E, K, Fe, and B12), and hypoalbuminemia,[3,18,19] making it difficult to diagnose. SIBO might be suspected in cases where vitamin deficiencies or weight loss seem to be outside the normal range, and other diagnoses are excluded. For example, while thiamine deficiencies are common following bariatric surgeries, two retrospective studies of 80 and 21 patients with obesity who underwent RNYGB reported that the presence of SIBO could explain post-operative thiamine deficiency accompanied by folate excess or unresponsiveness to supplementation, which are unusual.[20] Of the 80 patients in the first study, 39 (49%) had low thiamine levels, and of these, 28 (72%) had elevated serum folate. Meanwhile, high serum folate was more rare in the 41 patients with normal serum thiamine (p<0.01). The authors suggest that the combination of low serum thiamine and high serum folate might indicate SIBO. Indeed, 15 patients with low serum thiamine had positive hydrogen breath tests 30 minutes after an oral dose of glucose. The second study involved 21 patients with thiamine deficiency and evidence of SIBO by hydrogen breath testing. Oral thiamine supplementation alone failed to correct this deficiency, while oral antibiotics combined with thiamine supplements led to normal thiamine levels in nine patients.
In other cases, SIBO can present with more dramatic symptoms. In a case series of two female patients who had undergone RNYGB, elevated respiratory hydrogen tests were observed in conjunction with total body weight loss of 52 percent and 34 percent within 21 and 15 months of surgery, respectively, and was accompanied by asthenia, alopecia, edema, and hypoalbuminemia (to 24g/L and 34g/L, respectively).[21] Both patients received 30 days of ciprofloxacin and tetracycline antibiotic therapy, which led to resolution of their symptoms. While the retrospective nature of these studies and small sample sizes preclude drawing strong conclusions about the role of SIBO, clinical awareness of SIBO as a potential cause of persistent vitamin deficiencies and total protein malnutrition in patients who have undergone bariatric surgery can guide management toward hydrogen breath testing and antibiotic therapy. With proper therapy, symptomatic relief for these patients and prevention of the development of serious complications such as Wernicke encephalopathy can be achieved.[22,23]
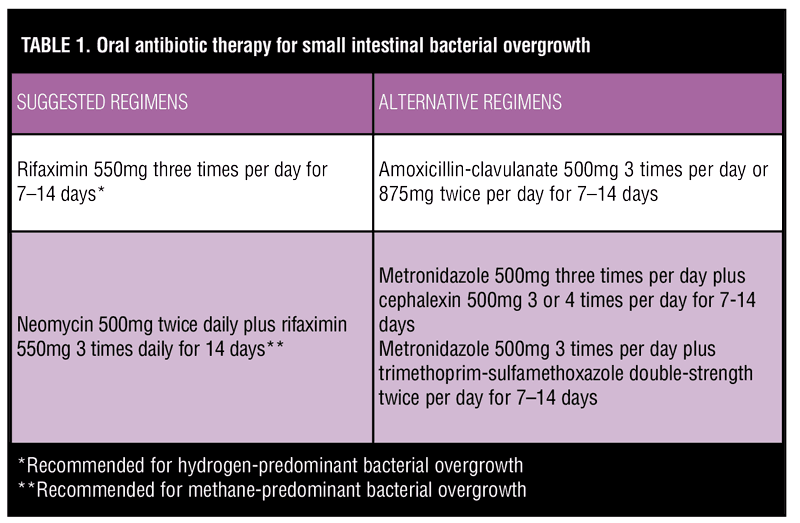
Because of the rarity of SIBO following RNYGB, optimal treatment is poorly understood. Management strategies include providing nutritional support, addressing the infection with antibiosis, or, in severe cases, correcting the underlying cause with revisional surgery.[9] The gold standard for treatment is antibiosis, but the choice of antibiotic regimen and duration of therapy remain controversial. Amoxicillin-clavulanate, ciprofloxacin, doxycycline, rifaximin, chlortetracycline, and metronidazole all have been used empirically in clinical practice, though studies directly comparing these regimens are sparse, especially in the setting of bariatric surgery. One randomized, controlled trial of 142 patients with SIBO found that rifaximin 1200mg/day for seven days was superior to metronidazole 750mg/day for seven days in reversing glucose breath test positivity at one month following randomization.[24] Suggested antibiotic regimens for treatment of SIBO are described in Table 1.[25–28]
The role of the gut microbiome in weight regulation following RNYGB outside of clinically symptomatic SIBO is unclear. One study of 65 patients found that post-operative patients with positive glucose breath tests experienced less weight loss than those with normal glucose breath tests[29]. However, the authors did not observe increased rates of symptoms of SIBO (e.g., diarrhea, vitamin deficiency, and bloating) in patients with positive tests, again emphasizing the difficulty of accurately diagnosing SIBO in the presence of alterations in orocecal transit time following RNYGB. A similar study of 33 patients found no difference in weight loss related to hydrogen breath test status.[30] Nevertheless, using unbiased high-throughput sequencing technologies,[31] RNYGB has been shown to have major effects on the small intestinal microbiome. In a randomized controlled trial of 44 patients undergoing RNYGB.[32] Probiotic therapy has been shown to reduce rates of positive hydrogen breath testing, promote weight loss, and increase vitamin B12 availability. Thus, while debilitating side effects of SIBO such as BADAS are now rare due to the replacement of JIB with more modern surgeries, changes in the small intestinal microbiome might have subtle but important effects on patients undergoing malabsorptive bariatric procedures.
Conclusion
Bariatric surgery creates major alterations in the composition and activity of microorganisms inhabiting the gut, which can have effects ranging from minor changes in long-term weight loss to, in rare cases, serious malnutrition and abdominal discomfort. Timely diagnosis of SIBO in post-surgical patients with abnormal gastrointestinal symptoms could guide management toward appropriate antibiotic therapy and prevent potential morbidity from long-term vitamin deficiencies, gastrointestinal upset, total protein malnutrition, or suboptimal weight loss. Further research investigating the relationship between microbiology and bariatric surgery could further elucidate mechanisms by which changes in the gut microbiome affect nutrient absorption and weight loss in bariatric surgery patients. Additional research will also contribute to the discovery of new surgical innovations and medical management strategies that lead to optimal patient outcomes.
References
- Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 366. 2012;1567–1576.
- Inge TH, Courcoulas AP, Jenkins TM, et al. Weight loss and health status 3 years after bariatric surgery in adolescents. N Engl J Med. 2016;374:113–123.
- Dukowicz AC, Lacy BE, Levine GM. Small intestinal bacterial overgrowth: a comprehensive review. Gastroenterol Hepatol. 2007;3:112–122.
- Khoshini R, Dai SC, Lezcano S, Pimentel M. A systematic review of diagnostic tests for small intestinal bacterial overgrowth. Dig Dis Sci 2008;53:1443–1454.
- Abidi WM, Chan WW, Thompson CC. Mo1285 breath testing for small intestinal bacterial overgrowth in Roux-en-Y gastric bypass patients: the importance of orocecal transit time. Gastroenterology. 2016;150:S688–S689.
- Griffen, WO Jr, Bivins BA, Bell RM. The decline and fall of the jejunoileal bypass. Surg Gynecol Obstet. 1983;157:301–308.
- McGouran RC, Rutter KP, Ang L, et al. Role of anaerobic bacteria in weight loss and reduced food intake after jejuno-ileal bypass in the rat. Int J Obes. 1982;6:197–204.
- Corrodi P, Jejunoileal bypass: change in the flora of the small intestine and its clinical impact. Rev Infect Dis. 1980; 6 Suppl 1:S80–84.
- Stein HB, Schlappner OL, Boyko W, et al. The intestinal bypass: arthritis-dermatitis syndrome. Arthritis Rheum 1981;24:684–690.
- Ely PH. The bowel bypass syndrome: a response to bacterial peptidoglycans. J Am Acad Dermatol. 1980;2:473–487.
- Jorizzo JL, Apisarnthanarax P, Subrt P, et al. Bowel-bypass syndrome without bowel bypass. Bowel-associated dermatosis-arthritis syndrome. Arch Intern Med. 1983;143:457–461.
- Ginsberg J, Quismorio FP Jr, DeWind LT, Mongan ES. Musculoskeletal symptoms after jejunoileal shunt surgery for intractable obesity. Clinical and immunologic studies. Am J Med. 1979;67:443–448.
- Drenick EJ, Ahmed AR, Greenway F, Olerud JE. Cutaneous lesions after intestinal bypass. Ann Intern Med 1980;93:557–559.
- Slater GH, Kerlin P, Georghiou PR, Fielding GA. Bowel-associated dermatosis-arthritis syndrome after biliopancreatic diversion. Obes Surg. 2004;14:133–135.
- Tu J, Chan JJ, Yu LL. Bowel bypass syndrome/bowel-associated dermatosis arthritis syndrome post laparoscopic gastric bypass surgery. Australasian Journal of Dermatology. 2011;52:e5–e7.
- Dicken CH. Bowel-associated dermatosis-arthritis syndrome: bowel bypass syndrome without bowel bypass. Mayo Clin Proc. 1984;59:43–46.
- Jorizzo JL, Schmalstieg FC, Dinehart SM, et al. Bowel-associated dermatosis-arthritis syndrome. Immune complex-mediated vessel damage and increased neutrophil migration. Arch Intern Med. 1984;144;738–740.
- Decker GA, Swain JM, Crowell MD, Scolapio JS. Gastrointestinal and nutritional complications after bariatric surgery. Am J Gastroenterol. 2007;102: 2571–2580;quiz 2581.
- Chan WW, Thompson CC, Lautz DB, Burakoff R. Risk of small intestinal bacterial overgrowth in Roux-en-Y gastric bypass. Gastroenterol. 2011;140;S-1057.
- Lakhani SV, Shah HN, Alexander K, et al. Small intestinal bacterial overgrowth and thiamine deficiency after Roux-en-Y gastric bypass surgery in obese patients. Nutr Res. 2008;28:293–298.
- Machado JD, Campos CS, Lopes Dah Silva C, et al. Intestinal bacterial overgrowth after Roux-en-Y gastric bypass. Obes Surg. 2008;18:139–143.
- Nautiyal A, Singh S, Alaimo DJ. Wernicke encephalopathy—an emerging trend after bariatric surgery. Am J Med. 2004;117:804–805.
- Salas-Salvado J, Garcia-Lorda P, Cuatrecasas G, et al. Wernicke’s syndrome after bariatric surgery. Clin Nutr. 2000;19:371–373.
- Lauritano EC, Gabrielli M, Scarpellini E, et al. Antibiotic therapy in small intestinal bacterial overgrowth: rifaximin versus metronidazole. Eur Rev Med Pharmacol Sci. 2009;13:111–116.
- Pimentel M, Chang C, Chua KS, et al. Antibiotic treatment of constipation-predominant irritable bowel syndrome. Dig Dis Sci. 2014;59:1278–1285.
- Attar A, Flourie B, Rambaud JC, et al. Antibiotic efficacy in small intestinal bacterial overgrowth-related chronic diarrhea: a crossover, randomized trial. Gastroenterology. 1999;117:794–797.
- Scarpellini E, Gabrielli M, Lauritano CE, et al. High dosage rifaximin for the treatment of small intestinal bacterial overgrowth. Aliment Pharmacol Ther. 2007;25:781–786.
- Singh VV, Toskes PP. Small bowel bacterial overgrowth: presentation, diagnosis, and treatment. Curr Gastroenterol Rep. 2003;5:365–372.
- Sabate JM, Coupaye M, Ledoux, et al. Consequences of small intestinal bacterial overgrowth in obese patients before and after bariatric surgery. Obes Surg. 2017;27:599–605.
- Andalib I, Shah H, Bal BS, et al. Breath hydrogen as a biomarker for glucose malabsorption after Roux-en-Y gastric bypass surgery. Dis Markers. 2015;2015:7.
- Kong LC, Tap J, Aron-Wisnewsky J, et al. Gut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genes. Am J Clin Nutr. 2013; 98:16–24.
- Woodard GA, Encarnacion B, Downey JR, et al. Probiotics improve outcomes after Roux-en-Y gastric bypass surgery: a prospective randomized trial. J Gastrointest Surg. 2009;13:1198–1204.
Category: Past Articles, Review




My wife had a normal hernia and tif repair now is experiencing severe vomiting daily. We left the hospital that preformed the surgery to go to Stanford. They have now told us she has small bacteria overgrowth In association with the tiff and hernia repair. Could be explained by potential operative. In tandem with likely antibiotics and acid suppressive therapy. And that they can’t fix her. What can I do to hell my wife she is dieing in front of my eyes every day at only 34 years old.
I would like to engage in research on this. Currently a size 1 and super fit but had this surgery twice. Diagnosed with SIBO and I believe it has colonized in the unused pouch. Possibly needing the surgery undone. I was able to change many lifestyle choices and corrected deficiencies but the abdominal distention is still horrific.