Management of the Contained Sleeve Leak
 by STEVEN G. LEEDS, MD, FACS, and MARC. A. WARD, MD
by STEVEN G. LEEDS, MD, FACS, and MARC. A. WARD, MD
Dr. Leeds and Ward are Assistant Clinical Professors of Surgery at Texas A&M College of Medicine in Bryan, Texas. Dr. Leeds is the Division Chief of Minimally Invasive Surgery, and Dr. Ward is Medical Director of Minimally Invasive Research and Simulation at Baylor University Medical Center at Dallas, Center for Advanced Surgery, Baylor Scott & White in Dallas, Texas.
FUNDING: No funding was provided.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: Laparoscopic sleeve gastrectomy has become the most common bariatric procedure performed since 2013. It appears to be safe and effective in both the short and long term. However, the most worrisome complication is a staple line leak. When patients are hemodynamically stable, there is likely a self- contained leak which can be difficult to manage. Nonsurgical approach is feasible, but a self-contained leak provides more options for treatment. Being well versed in all therapeutic options will improve the surgeon’s ability to manage these complicated leaks. Prior to starting management, it is necessary to identify the leak site with the correct imaging and understand the proposed classifications for help in deciding on a management plan. Nonoperative, endoscopic, and surgical options are available. Popular endoscopic options for management include a self-expanding stent, endoluminal vacuum therapy, endoscopic internal drainage, and endoscopic septotomy. Surgical options include esophago-jejunostomy, fistula-jejunostomy, or Roux-en-Y gastro-jejunostomy. All have their merits and we present review of the literature and our preferred management algorithm as a high-volume facility managing more than 40 sleeve leaks. Management of these patients needs to be done in a facility with experienced surgeons who are trained to offer most, if not all, options.
KEYWORDS: Sleeve gastrectomy leak, endoscopic surgery, bariatric surgery complications
Bariatric Times. 2020;17(4):9–11.
Laparoscopic sleeve gastrectomy (SG) has become the most common bariatric procedure performed since 2013.¹ It appears to be safe and effective in both the short and long term.²,³ However, the growing popularity of this relatively new procedure has introduced the surgical community to a new set of side effects and complications that can be difficult to manage. These include gastroesophageal reflux disease (GERD), weight recidivism, dysphagia, and staple line leaks, the latter of which is arguably the most worrisome.4,5 The reported leak rate for SG is 1 to 3 percent,6–8 and with rising popularity and increasing number of cases, leak management has become a significant concern and an area of interest. The hemodynamically unstable patient should be dealt with immediately with surgical exploration to gain control of the source. However, there is likely a self-contained leak, which can be difficult to manage. Here, a nonsurgical approach is described that provides more options for treatment. Being well-versed in all therapeutic options will improve the surgeon’s ability to manage complicated leaks following SG.
LEAK IDENTIFICATION
There are important characteristics about a SG leak that should be noted to manage it correctly. A stepwise algorithm for the important points
of leak identification and classification prior to proceeding with management are seen in Figure 1. The most common modalities include upper GI series (UGI) and/or computed tomography (CT) to identify the leak and the associated cavity. CT scans should be done with oral and intravenous contrast, if possible, to accurately identify the degree of contamination and size of the abscess cavity. UGI can also be used to the find the leak; however, it should be understood that the sensitivity to detect the leak is variable due to size and location of the defect. When undergoing the UGI series, multiple positions of the patient might be required in opposing gravity to accurately identify the site of the leak. The most important modality is upper endoscopy, but this should be done by an expert endoscopist and ideally at the time of intervention.
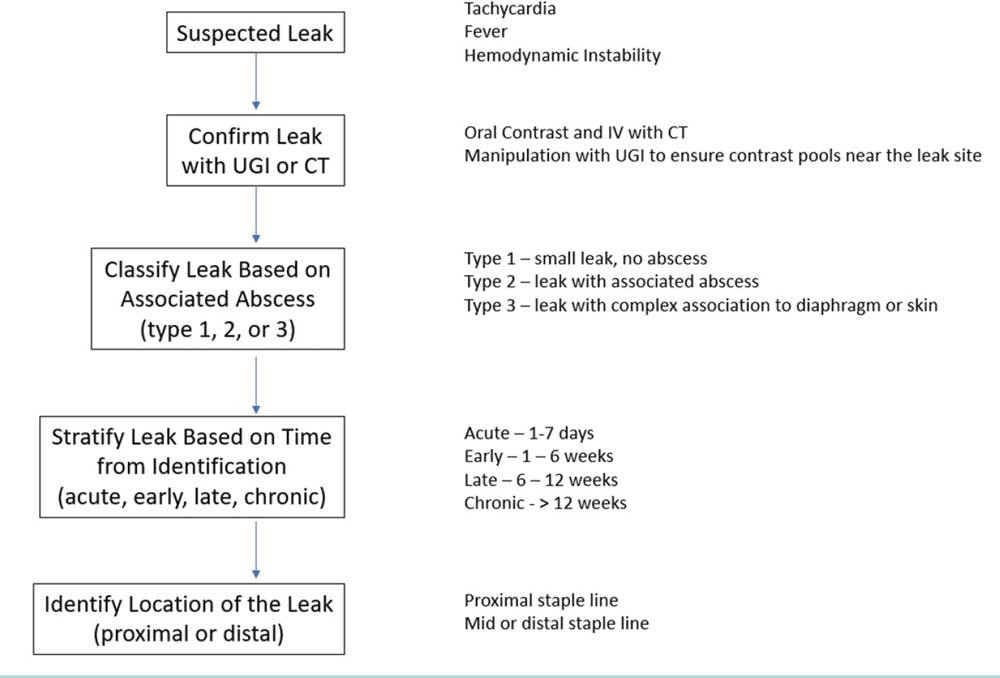
FIGURE 1. Decision tree including information that needs to be ascertained when treating the SG leak
While there is no uniform classification systme currently available, the following classification appears to demonstrate the best differentiation. Type I fistulas include a tiny leak without an associated abscess, Type 2 fistulas include a leak with an associated contained abscess cavity, and Type 3 fistulas include a leak with complexity extending subdiaphragmatic or an external gastrocutaneous fistula. Additionally, recognition of a SG leak in its relation to the sentinel surgery is a key component in determining the best management technique where surgical, endoscopic, and nonprocedural techniques should be implemented. Classification of the sleeve leak occurrence can fall into one of four categories based on how long after surgerey the SG leak was recognized; acute is within the first seven days, early is within 1 to 6 weeks, late is within 6 to 12 weeks, and chronic is beyond 12 weeks.7
Lastly, it is important to note the location of the leak along the staple line. Greater than 70 percent of SG leaks occur at the proximal staple line, indicating there might be an explainable cause.10, 11 For example, in half of the cases, the vertical staple line changed the blood flow of
the sleeve, creating weakness in the proximal portion.12 Other researchers have suggested improper staple height in this area might lead to leaks due to the variation in the thickness of the tissue throughout the stomach.13 Finally, excessive intraluminal pressure within the sleeve can shift the focus of pressure proximally.14Ultimately, it is likely that all these factors contribute, and any stenosis in the gastric tube will increase the chances of a leak.
SURGICAL INTERVENTION
Once the decision is made to proceed to the operating room for a contained leak, the options fall into two categories. The first option is to perform a washout and wide drainage to remove contaminated fluid and transform the leak site into a controlled fistula to the skin. Attempts can be made to surgically repair the leak at this time, but this is not always necessary, and it often fails. The second option is definitive management with a gastrectomy and esophago-jejunostomy (EJ), a fistula-jejunostomy (FJ) with preservation of the continuity of sleeve anatomy, or conversion to a Roux-en-Y gastric bypass (RYGB) where the leak is located either mid to distal sleeve. In the case of an EJ, the gastric tube is typically deformed and causing the distal obstruction that precipitated the SG leak. In the case of a FJ, the leak site is amenable to an enteric drainage procedure. This will preserve the normal anatomy and allow for continued weight loss.15,16 The decision between the two procedures needs to be done by an experienced surgeon due to the technical complexity, as well as the postopereative management. In some cases, esophagectomy with proximal gastrectomy might be needed, but usually this is only required in cases of significant contamination with or without hemodynamic instability.
ENDOSCOPIC INTERVENTION
Self-expanding stents (SES). The most common endoscopic modality currently used to treat SG leaks are self-expanding stents (SES). The advantage for using SES is their ability obtain source control. Selecting the appropriate stent size will create radial pressure in the lumen to fix the stent in the desired location, creating a path for flow within the lumen of the stent for enteric contents to flow blocking any leakage. Fully covered and partially covered stents should be used. The disadvantage to SES is that it doesn’t provide any resolution for the contaminated tissue already affected as well as potential abscess cavities adjacent to the leak site. Often, surgical washout or percutaneous drainage are needed to obtain source control. Another disadvantage of SES in the instance of an SG leak is the high migration rate due to the location of the leak site. High instances of leaks at the proximal staple line requires the proximal end of the stent to be placed in the distal esophagus, which can lead to strictures and is uncomfortable for the patient, resulting in nausea, pain, and GERD.
Aside from SES’s ability to protect the leak site, there is another often-overlooked advantage. SES can also be used to help with any gastric tube deformities. With SG leaks, the gastric tube is tortuous or has a stenotic portion. Use of SES can help open these deformities to facilitate healing because of re-established outflow. Using SES in this manner often doesn’t account for the ongoing contamination but can be used when the abscess cavity has already been decontaminated. For example, after the cessation of another technique, SES can be used to dilate a stricture through the rest of the healing process.
A review of the literature for SES and SG leaks revealed several studies but were of relatively low patient numbers. A study that included 67 SG leaks showed 88-percent success rate of SES usage.17 The authors also did an analysis of their own 17 patients, showing a 76-percent success rate, with a median 42 days dwell time and a six-percent migration rate. There were various types of stents used in the review, but the success rate range in the 18 studies wasn’t big enough to indicate that one stent was superior over another. All stents were either fully covered or partially covered. Subset analysis did identify that the longer duration of a SG leak decreased the ability to heal the perforation with a SES. Best success with SES treatment was noted in leaks less than seven days old. A similar finding was seen in the consensus statement that SG leaks longer than 30 days should not undergo endoscopic management with SES because the chance of healing is low.6 A meta-analysis and systematic review included 187 patients with a similar healing rate at 72 percent, mean 49 days dwell time, and a 28-percent migration rate. 18 Another study of a novel stent to improve these results was done to include 37 patients with a continued heal rate of 78 percent, mean dwell time of 29 days, and a migration rate of 22 percent. It appears that, despite the type of stents, the rate of healing is roughly 75 percent, with the most frequent complication is stent migration occurring nearly 25 percent of the time.
Endoluminal vacuum therapy (EVAC). EVAC therapy is an emerging technique that provides several advantages. It serves to obtain and maintain source control. The purpose of the endosponge is to adhere to a large surface area and block further leakage of enteric contents and adds negative pressure to the contaminated tissue to promote decontamination and debridement. The negative pressure will also aid to reperfuse the contaminated tissue facilitating healing. EVAC works so well at decontamination, that it is advisable to remove any percutaneous drains to prevent redundant foreign bodies in the wound bed harboring a large bacterial load. These advantages allow this procedure to be used in patients who are hemodynamically unstable in experienced hands because it works well to control the source of the sepsis. The disadvantage of EVAC therapy is that it is a wound dressing that needs to be changed frequently, and, to remove the endosponge, the patient needs to undergo sedation to have it removed endoscopically. Patients typically undergo several subsequent procedures, which often means they will remain inpatient. While this can be a potential disadvantage, it does add the advantage of providing an ongoing wound inspection where progress of healing can be assessed.
EVAC therapy for SG leaks was first done in 2016, where nine patients underwent the procedure with a 89-percent healing rate over a mean of 51 days.19 This was a heterogeneous group where prior to EVAC therapy, four patients had over-the-scope clips (OTSC) placed, six had prior laparoscopy or laparotomies, and six had SES use. Follow-up studies from the same institution showed 17 patients with a 82-percent healing rate over a mean of 56 days,20 and then 24 patients with a 79-percent healing rate over a mean of 44 days.21 An alternate study in Germany was done with six patients showing a 100-percent healing rate over a median of 24 days.22 The use of EVAC therapy is gaining popularity, and more data will emerge in the field of managing bariatric surgery leaks.
Endoscopic internal drainage (EID). Postoperative fluid collections can often be drained percutaneously, but SG leaks typically present unique collections that are difficult to access percutaneously due to anatomical barriers (i.e., liver, spleen, lung). EID works to place the catheter endoscopically and have it drain into the lumen of the GI tract since there is direct communication to a cavity. This can be done under direct visualization or under fluoroscopic guidance based on the endoscopist’s preference. The advantage of EID is that it avoids the need to manage a percutaneous drain and avoids a gastrocutaneous fistula tract. The healing process will collapse the cavity, making it contiguous with the lumen. Correct patient selection is key to its success with some cavities having a large leak defect, which will make the fixation of the drain difficult, so it should be used in the instances where a cavity is found, as seen in the Type 2 leaks.
EID was first introduced using biliary double-J pigtail stents.23, 24 One study of 25 patients reported a 96-percent healing rate using this technique in these types of SG leaks.25 Enterocutaneous or gastropleural fistulas tend to respond poorly to this technique, and the authors recommend surgical intervention in these circumstances. Other studies have shown similar results reaching 75-to-100-percent healing rates,26, 27 and a recent systematic review of 385 patients reported in the literature had an overall heal rate of about 83 percent.28 Ultimately, identifying the correct patient for EID will predict its best outcome.
Septotomy. In more mature fistula cavities where the abscess has epithelialized, and outflow of the cavity is now obstructed, a septotomy can be used. This essentially marsupializes the cavity into the natural lumen to facilitate drainage. Its advantage is that it requires one single procedure by an experienced endoscopist. Its disadvantage is that it has a narrow spectrum of SG leaks where it can be used. These are typically late or chronic leaks characterized as a Type 2 leak. This should not be used in instances where a well-defined cavity has not been created.
The first large report was for 27 patients where serial procedures were used to open the SG leak site via septotomy.29 It needs to be emphasized that these cases are complicated by a distal obstruction that should be addressed with endoscopic dilations that contribute to the serial procedures. Subsequent cohorts have shown promise with this techniques in abscess cavities up to 10cm.30 The chronicity of the SG leak is important and has been shown to be effective in patients failing other modalities where the leak persists greater than six months.31 Other institutions have been able to reproduce similar outcomes but also emphasize the need to dilate the distal stricture.32
Other techniques. There are other available techniques, including over-the-scope clips (OTSC) and fibrin glue, but the reports are few, and further studies are needed to define their role in managing SG leaks.
NONPROCEDURAL INTERVENTION
Nonprocedural intervention occurs when the leak resolves spontaneously and resides in a subclinical manner. There have been reports in hemodynamic stable patients where the leak resolves after starting antibiotics, put on total parenteral nutrition (TPN), and kept without oral intake. Success is low in one study only reporting a 25-percent healing rate in eight patients, while 75 percent of patients required SES placement to resolve the leak.33 Another study reports one patient undergoing nonprocedural management in a series of six patients with a leak and healed in 33 days.34 This method should be reserved for low acuity patients who can be observed for clinical decompensation, and in a location where endoscopic and surgical modalities are available. Prolongation of this method without expert surveillance can create a Type 2 or 3 leak that will need more aggressive management. This should only be done in a narrow spectrum of patients and likely where the gastric tube has been verified as unobstructed, so that outflow is not impeded.
COMBINATION OF THERAPIES
Combining therapies is a useful strategy. There is a large gap in the literature about when to use each of these modalities, which is the aim of this article. These techniques can either be used concomitantly, or subsequently on the same patient. Recognition of the right combination and timing comes with experience, and a thorough understanding of the individual techniques is needed to prevent counterproductive attempts at healing. For example, SES and EVAC used at the same time is not recommended. SES has a mechanism of action that requires the lumen to be fixed open (while excluding the perforation cavity), and EVAC has a mechanism of action that requires the lumen to be collapsed and sealed. Use together will cause ongoing leak with further contamination. But, using SES and EID together can prove to be useful to drain a fistula cavity while stenting open a distal stricture (Figure 2).
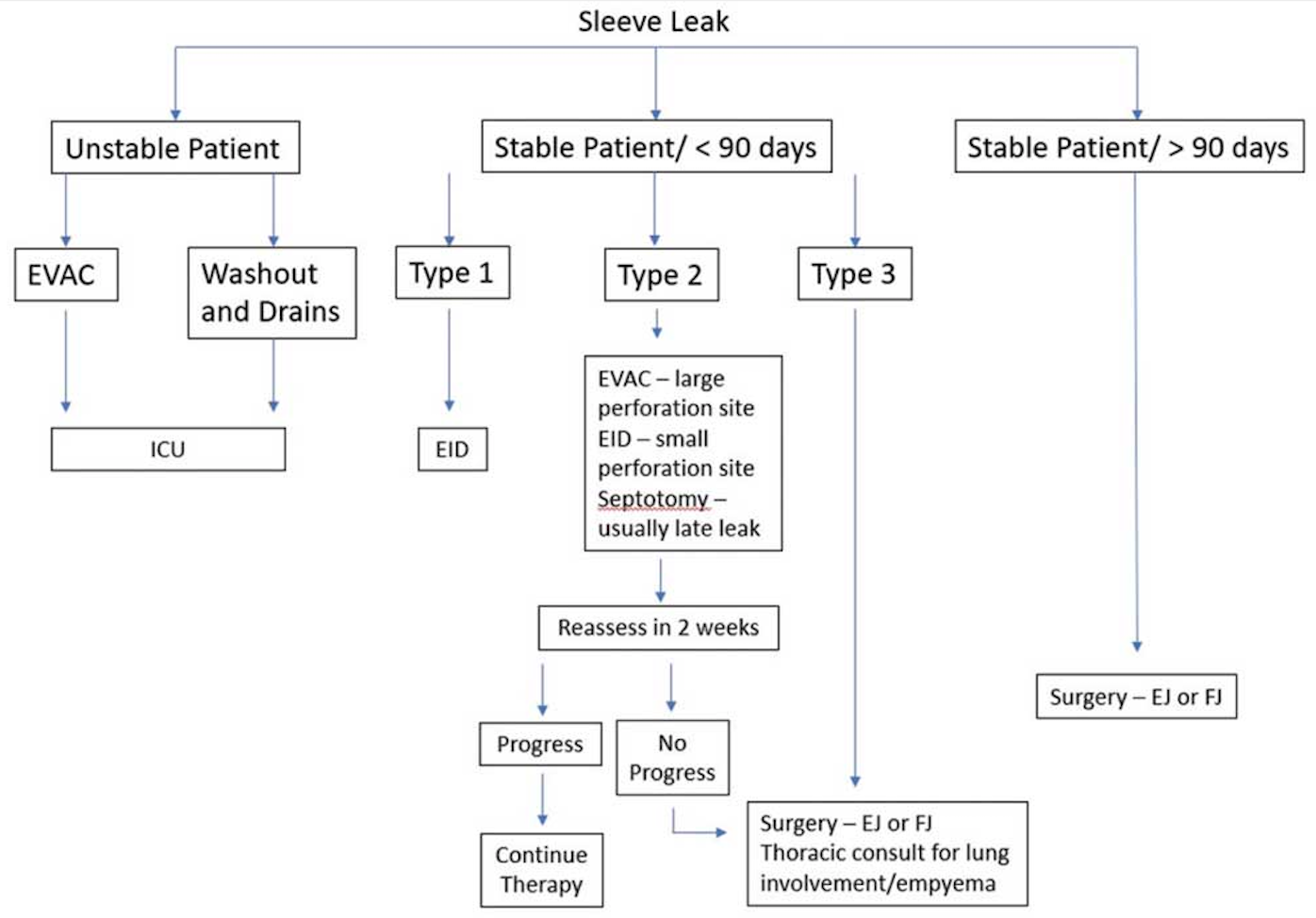
FIGURE 2. Self expanding stent (SES) and endoscopic internal drainage (EID) in combo therapy. There is a stent placed to dilate the sleeve stricture. The pigtail stent is shown proximal to the stenosis through the perforation site. The pigtail does not need to be excluded with the SES despite being shown here.
One of the biggest hurdles is to determine when true progress is being made or whether a particular modality isn’t working and needs to be transitioned to something different. Using these techniques subsequently, and not together, is useful. For example, in cases of SES use, migration rate tends to be high, and when this occurs, a different modality needs to be instituted. Transition to a new technique, such as EID or EVAC, might prove to be more efficacious. This represents a care model that needs to be continually monitored for change and improvement. If this is not available at a current institution, then the patient should be transferred.
ALGORITHMS
Several algorithms exist for outlining a care pathway for managing a SG leak.9, 30, 35, 36 None are the same, but there are distinct commonalities. First, it is not questioned that clinically unstable patients with a SG leak need surgical intervention along with recommendations for resuscitation and broad-spectrum antibiotics. Second, the best evaluation methods are CT of the abdomen and pelvis and UGI. Endoscopy is a modality that is commonly listed as a means for treatment and not necessarily for diagnosis. There is also emphasis on looking for stenosis or deformation of the sleeve, as well as the most-common modality of using SES. Lastly, surgery for diversion, resection, or attempt at repair seems to be the last resort. In addition to all these commonalities, it needs to be emphasized that nutritional status must be assessed, as well as provide nutrition either parenternally or enterally for optimal outcome.
The appearance of the postsleeve anatomy is likely the most important factor in deciding which pathway to choose for management of a SG leak. Major deformations in sleeve anatomy often hinder the ability of endoscopic interventions from closing the defect and can contribute to dysphagia and GERD, even if the leak eventually heals. In cases like these, patients should be taken for surgical intervention rather than wasting time with endoscopic therapies.
MANAGEMENT IN AN EXPERIENCED CENTER
Probably the biggest difficulty with SG leaks and endoscopic management is that it represents a heterogenous group. All leaks are different and could have been identified at different points in their natural history. Other modalities have usually been attempted prior to transfer, such as surgical intervention, which have failed. This makes it difficult to study SG leaks and form a consensus protocol on management. A great example is the use of OTSC to heal SG leaks. It was shown in a large systematic review that there is merit to this modality, but the patient population was so heterogenous, and multiple other procedures had been attempted prior to OTSC use, so it is hard to interpret true results to find the proper indication.37 In any study evaluating endoscopic success, the usual outcome is around an 80-percent success rate, regardless of which modality is used. It is important to understand that all modalities have certain instances where their success is predicated on the situation. For example, endoscopic treatment of SG leaks hasn’t shown significant promise when leaks are chronic. In this instance, patients should be pushed to surgical intervention avoiding a long, drawn-out endoscopic course resulting in malnutrition, muscle wasting, and psychiatric burden. However, there is no consensus on the timing and use of these endoscopic modalities. As a consequence, the best opportunity for success in healing these patients is their admission to an experienced center and managed in a timely fashion. A center that has experience in managing SG leaks will have the ability to offer a variety of endoscopic and surgical options.
OUR CURRENT APPROACH
Our current institutional management of SG leaks assesses the stability of the patient and the chronicity of the leak (Figure 3). We have a significant experience in our facility with over 40 SG leaks managed.
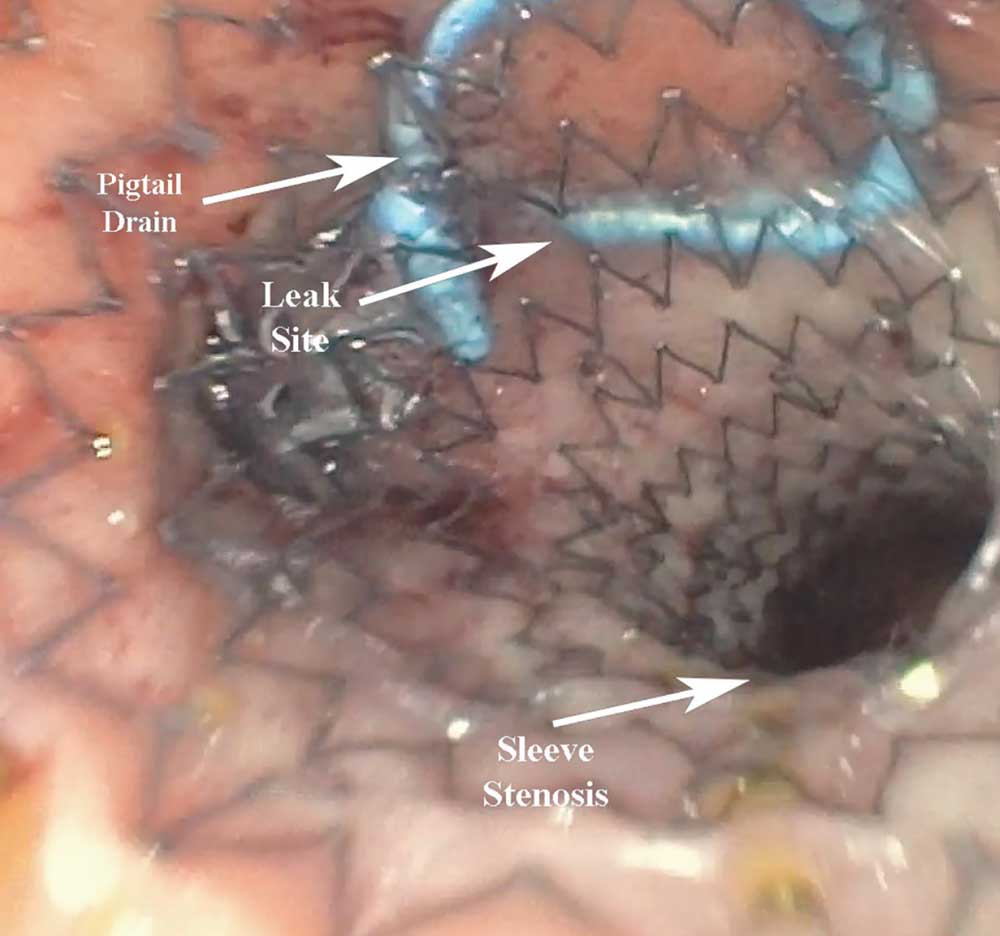
FIGURE 3. Current institutional algorithm for managing SG leaks
The unstable patient. Hemodynamic stability is the first decision point in the algorithm to understand the immediate need for intervention. Given our institution’s extensive use of EVAC therapy and its ability to gain source control, we will use this in the unstable patient. This is not recommended in institutions with minimal or no experience. In this case, patients will either undergo washout and drainage laparoscopically, or have EVAC started. They are admitted to the intensive care unit (ICU), and resuscitation begins with intravenous antibiotics.
The stable patient—leak detected <90 days. Evaluation is done with UGI and CT scan. The CT scan will provide information about the cavity where the UGI will help identify the location. Small perforations (<1cm) with large cavities are managed with a wide variety of options, whereas a large perforation (>1cm) and a small cavity are typically managed with EVAC. Large perforations regardless of the cavity size typically start with EVAC. The progress in therapy is monitored, and the modality is changed after 2 to 3 weeks if there is regression or delay of healing. If the perforation is small (< 1cm), EID or septotomy is generally used. EVAC therapy with an intraluminal endosponge placement or SES occluding the small perforation site can be attempted, but we have found them to be poorly effective. These therapies usually get 2 to 4 weeks of treatment and are reassessed. If the patient continues to fail endoscopic therapy, the patient is taken for definitive EJ or FJ.
The stable patient—leak detected >90 days.
It is our finding, and is consistent with the literature, that endoscopic modalities have poor efficacy and that patients should be taken for definitive EJ or FJ. Certain cases could be evaluated for septotomy.
CONCLUSION
The management of a contained SG leak is a topic that has not reached consensus. There are several ways to manage these patients that can include nonoperative, endoscopic, or surgical options. However, the most important part is that patients are treated in centers with expertise and those who offer most of the modalities, if not all of them. This will provide the patient with the best chance at healing the perforation by reducing their risk of additional morbidity and mortality.
REFERENCES
- Khorgami Z, Shoar S, Andalib A, et al. Trends in utilization of bariatric surgery, 2010-2014: sleeve gastrectomy dominates. Surg Obes Relat Dis. 2017;13(5):774–778.
- El Chaar M, Stoltzfus J, Melitics M, et al. 30-day outcomes of revisional bariatric stapling procedures: first report based on MBSAQIP data registry. Obes Surg. 2018;28(8):2233–2240.
- Golzarand M, Toolabi K, Farid R. The bariatric surgery and weight losing: a meta-analysis in the long- and very long-term effects of laparoscopic adjustable gastric banding, laparoscopic Roux- en-Y gastric bypass and laparoscopic sleeve gastrectomy on weight loss in adults. Surg Endosc. 2017;31(11):4331–4345.
- Clapp B, Wynn M, Martyn C, et al. Long term (7 or more years) outcomes of the sleeve gastrectomy: a meta-analysis. Surg Obes Relat Dis. 2018;14(6):741–747.
- Genco A, Soricelli E, Casella G, et al. Gastroesophageal reflux disease and Barrett’s esophagus after laparoscopic sleeve gastrectomy: a possible, underestimated long-term complication. Surg Obes Relat Dis. 2017;13(4):568–574.
- Gagner M, Hutchinson C, Rosenthal R. Fifth International Consensus Conference: current status of sleeve gastrectomy. Surg Obes Relat Dis. 2016;12(4):750–756.
- Rosenthal RJ, International Sleeve Gastrectomy Expert Panel, Aceves Diaz A, et al. International Sleeve Gastrectomy Expert Panel Consensus Statement: best practice guidelines based on experience of >12,000 cases. Surg Obes Relat Dis. 2012;8(1):8–19.
- Knapps J, Ghanem M, Clements J, Merchant AM. A systematic review of staple-line reinforcement in laparoscopic sleeve gastrectomy. JSLS. 2013;17(3):390–399.
- Al Hajj G, Chemaly R. Fistula following laparoscopic sleeve gastrectomy: a proposed classification and algorithm for optimal management. Obes Surg. 2018;28(3):656–664.
- Burgos AM, Braghetto I, Csendes C, et al. Gastric leak after laparoscopic-sleeve gastrectomy for obesity. Obes Surg. 2009;19(12):1672–1677.
- Aurora AR, Khaitan L, Saber AA. Sleeve gastrectomy and the risk of leak: a systematic analysis of 4,888 patients. Surg Endosc. 2012;26(6):1509–1515.
- Perez M, Hussain A, El-Hasani S, et al. Does anatomy explain the origin of a leak after sleeve gastrectomy? Obes Surg. 2014;24(10):1717–1723.
- Rawlins L, Rawlins MP, Teel D 2nd. Human tissue thickness measurements from excised sleeve gastrectomy specimens. Surg Endosc. 2014;28(3):811–814.
- Marie L, Masson C, Gaborit B, et al. An experimental study of intraluminal hyperpressure reproducing a gastric leak following a sleeve gastrectomy. Obes Surg. 2019;29(9):2773–2780.
- Chouillard E, Younan A, Alkandari M, et al. Roux- en-Y fistulo-jejunostomy as a salvage procedure in patients with post-sleeve gastrectomy fistula: mid-term results. Surg Endosc. 2016;30(10):4200– 4204.
- Szymanski K, Ontiveros E, Burdick JS, et al. Endolumenal vacuum therapy and fistulojejunostomy in the management of sleeve gastrectomy staple line leaks. Case Rep Surg. 2018;2018:2494069.
- Alazmi W, Al-Sabah S, AlMohammad Ali D, Almazeedi S. Treating sleeve gastrectomy leak with endoscopic stenting: the Kuwaiti experience and review of recent literature. Surg Endosc. 2014;28(12):3425–3428.
- Okazaki O, Bernardo WM, Brunaldi VO, et al. Efficacy and safety of stents in the treatment of fistula after bariatric surgery: a systematic review and meta-analysis. Obes Surg. 2018;28(6):1788– 1796.
- Leeds SG, Burdick JS. Management of gastric leaks after sleeve gastrectomy with endoluminal vacuum (E-Vac) therapy. Surg Obes Relat Dis. 2016;12(7):1278–1285.
- Mencio MA, Ontiveros E, Burdick JS, Leeds SG. Use of a novel technique to manage gastrointestinal leaks with endoluminal negative pressure: a single institution experience. Surg Endosc. 2018;32(7):3349–3356.
- Leeds SG, Mencio M, Ontiveros E, Ward MA. Endoluminal vacuum therapy: how I do it. J Gastrointest Surg. 2019;23(5):1037–1043.
- Morell B, Murray F, Vetter D, et al. Endoscopic vacuum therapy (EVT) for early infradiaphragmal leakage after bariatric surgery-outcomes of six consecutive cases in a single institution. Langenbecks Arch Surg. 2019;404(1):115–121.
- Slim, R, Smayra T, Noun R. Biliary endoprosthesis in the management of gastric leak after sleeve gastrectomy. Surg Obes Relat Dis. 2013;9(3):485– 486.
- Pequignot A, Fuks D, Verhaeghe P, et al. Is there a place for pigtail drains in the management of gastric leaks after laparoscopic sleeve gastrectomy? Obes Surg. 2012;22(5):712–720
- Sportes A, Aireini G, Kamel R, et al. Efficacy of endoscopic treatment of post-sleeve gastrectomy fistulas according to the radiological type. Obes Surg. 2019;29(7):2217–2224.
- Donatelli G, Ferretti S, Vergeau BM, et al. Endoscopic internal drainage with enteral nutrition (EDEN) for treatment of leaks following sleeve gastrectomy. Obes Surg. 2014;24(8):1400–1407.
- Talbot M, Yee G, Saxena P. Endoscopic modalities for upper gastrointestinal leaks, fistulae and perforations. ANZ J Surg. 2017;87(3):171–176.
- Giuliani A, Romano L, Marchese M, et al. Gastric leak after laparoscopic sleeve gastrectomy: management with endoscopic double pigtail drainage. A systematic review. Surg Obes Relat Dis. 2019;15(8):1414–1419.
- Baretta G, Campos J, Correia S, et al. Bariatric postoperative fistula: a life-saving endoscopic procedure. Surg Endosc. 2015;29(7):1714–1720.
- Mahadev S, Kumbhari V, Campos JM, et al. Endoscopic septotomy: an effective approach for internal drainage of sleeve gastrectomy-associated collections. Endoscopy. 2017;49(5):504–508.
- Campos JM, Ferreira FC, Teixeira AF, et al. Septotomy and balloon dilation to treat chronic leak after sleeve gastrectomy: technical principles. Obes Surg. 2016;26(8):1992–1993.
- Guerron AD, Ortega CB, Portenier D. Endoscopic abscess septotomy for management of sleeve gastrectomy leak. Obes Surg. 2017;27(10):2672– 2674.
- Moon RC, Shah N, Teixeira AF, et al. Management of staple line leaks following sleeve gastrectomy. Surg Obes Relat Dis. 2015;11(1):54–59.
- Musella M, Milone M, Bianco P, et al. Acute leaks following laparoscopic sleeve gastrectomy: early surgical repair according to a management algorithm. J Laparoendosc Adv Surg Tech A. 2016;26(2):85–91.
- Nedelcu M, Manos T, Cotirlet A, et al. Outcome of leaks after sleeve gastrectomy based on a new algorithm adressing leak size and gastric stenosis. Obes Surg. 2015;25(3):559–563.
- Nimeri A, Ibrahim M, Maasher A, Al Hadad M. Management algorithm for leaks following laparoscopic sleeve gastrectomy. Obes Surg. 2016;26(1):21–25.
- Shoar S, Poliakin L, Khorgami Z, et al. Efficacy and safety of the over-the-scope clip (OTSC) system in the management of leak and fistula after laparoscopic sleeve gastrectomy: a systematic review. Obes Surg. 2017;27(9):2410–2418.
Category: Past Articles, Review




