Nutrient Deficiencies Involved in the Development of Neurological Conditions after Metabolic and Bariatric Surgery
 This activity expired October 1, 2022.
This activity expired October 1, 2022.
Tracy Martinez, RN, BSN, CBN
Tracy Martinez, RN, BSN, CBN, is Program Director of Wittgrove Bariatric Center in Del Mar, California
A Message from the Department Editor
Dear Colleagues:
I hope this finds you well in this trying time of COVID-19. I am grateful for Cassie’s article, particularly in the absence of conferences. In my 28 years as a bariatric nurse, I have only seen a handful of neurological symptoms in our metabolic and bariatric surgical patients; however, as a clinician in our specialty, it is important to learn about all potential risks, signs and symptoms, as well as prevention and treatments. Without astute assessment and follow up, the conditions can be devastating.
I hope you learn a lot from this thorough and detailed article about nutrient deficiencies involved in the development of neurological conditions after metabolic and bariatric surgery.
My best to you,
Tracy Martinez, RN, BSN, CBN
by Cassie I. Story, RDN
Ms. Story has a private practice in Scottsdale, Arizona.
FUNDING: This continuing education activity was supported by Bariatric Advantage (Aliso Viejo, California).
DISCLOSURES: Cassie I. Story, RDN, is the Director of Nutrition Education for Bariatric Advantage.
ABSTRACT: There is an urgent need for science-based nutrition recommendations following metabolic and bariatric surgery. While evidence indicates that surgery is the most effective treatment option for someone affected by obesity, the increase in reported nutrition deficiencies and complications due to inadequate nutrition status postoperatively is cause for alarm. In this final part of a four-part Core 4 educational series on identifying, preventing, and treating nutrition deficiencies in preoperative and postoperative metabolic and bariatric surgery patients, we focus the micronutrient deficiencies that can impact neurological health.
KEYWORDS: Neurological conditions, thiamin, nutrient deficiencies, copper
Bariatric Times. 2020;17(10):10–15.
Nutritional deficiencies that impact the nervous system have been studied for over 100 years. In the 1800s, beriberi reached epidemic proportions around the world. Prior to 1940, pellagra was common in the southern United States, until enriching grain products with niacin and other B-vitamins became standard. Now, in developed countries, typical causes of nutrient deficiencies that impact neurological health include alcoholism, anorexia, obesity, a high carbohydrate intake, and impaired nutrient absorption secondary to intestinal malabsorption syndromes.1
Although rare, neurological conditions after metabolic and bariatric surgery (MBS) can lead to irreversible consequences and even death.2,3 Neurological conditions (see Table 1) can affect both the central and peripheral nervous systems, can present with clinical signs and symptoms (SS) in the absence of out-of-range serum marker detection, and can occur anywhere from three weeks to 20-plus years after surgery.5–7 The most acute nutrient deficiency, Wernicke encephalopathy (WE), has been reported in a range of surgical procedures including: Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy (SG), biliopancreatic diversion with or without duodenal switch (BPD-DS), gastric banding, gastrojejunostomy, and intragastric ballooning.8 Due to these factors, clinician awareness of these conditions is paramount to protect the health and wellbeing of the patient.
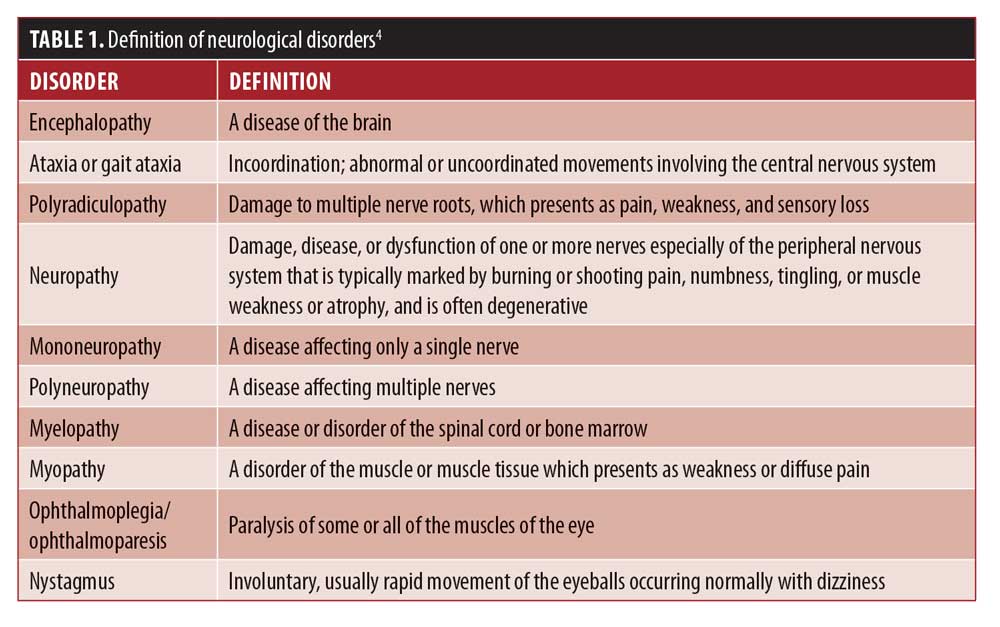
Unfortunately, for many patients who have had MBS, it is a matter of when they will become deficient in one or more micronutrients, not if.9 Healthcare professionals (HCPs) should be aware that the majority of neurological conditions that appear take years, and sometimes even decades, to develop, and they are often somewhat difficult to diagnose.10,11 Clinical history, micronutrient supplementation intake, physical SS, weight loss history, biochemical findings, magnetic resonance imaging (MRI), nerve transmission studies, biopsies, and response to therapy will all play a role in the accurate diagnosis and treatment of patients who present with neurological conditions after MBS.11 Prevention of these often irreversible conditions should be the goal of all HCPs and patients.12
Trends in clinical features of patients who present with neurological disorders following MBS include more rapid or absolute amount of weight loss, gastrointestinal (GI) issues, especially prolonged vomiting (but also dysphagia, and gastro-esophageal reflux), poor dietary intake, and lack of or inadequate micronutrient supplementation.11,13,14 Some neurological disorders can be attributed to a single micronutrient deficiency, such as thiamin deficiency leading to WE. However, many are the result of multiple micronutrient deficiencies.7
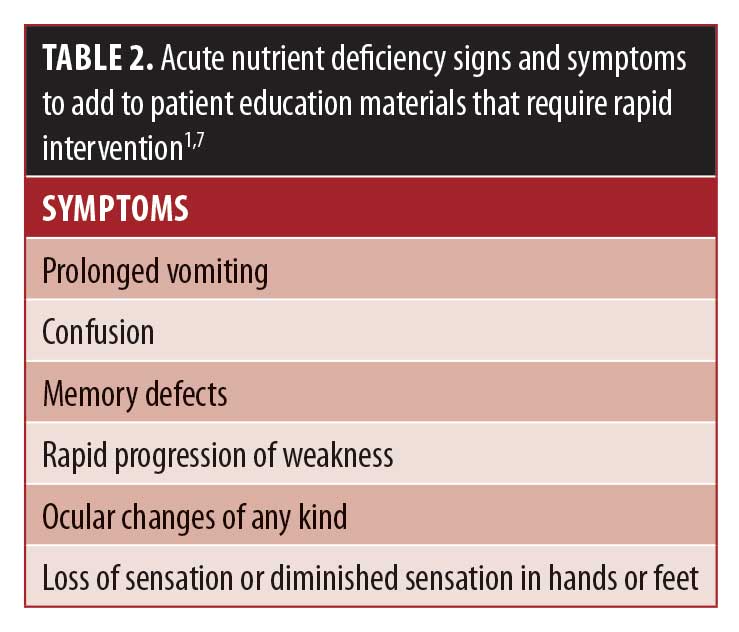
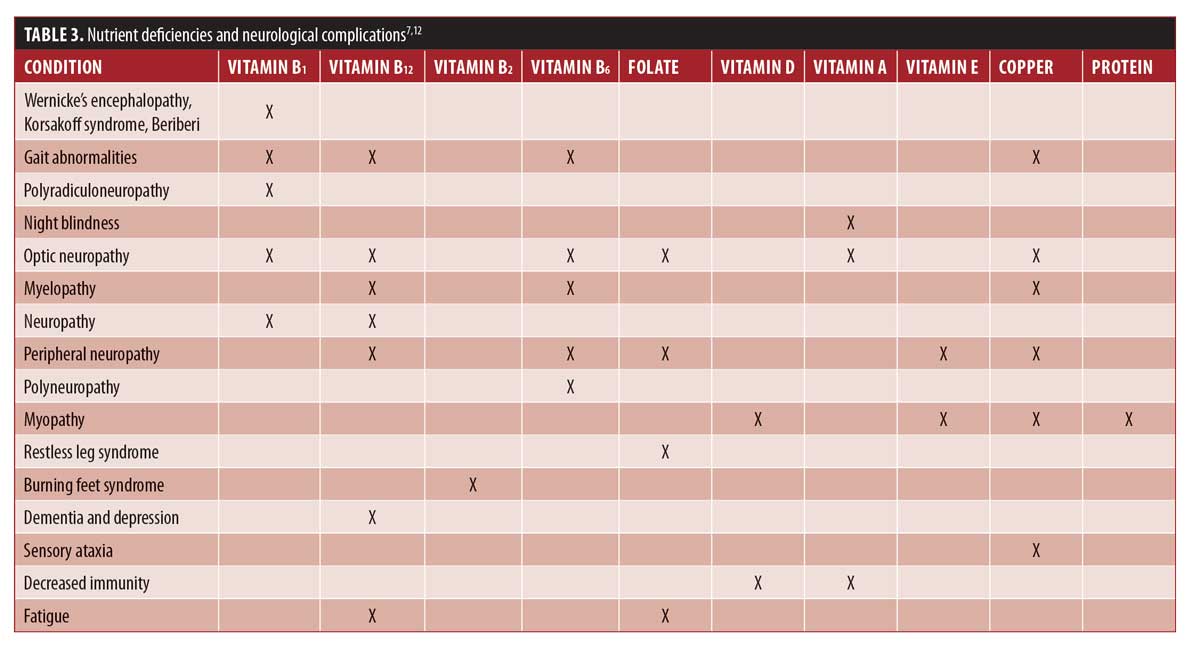
While some micronutrient deficiency SS are subtle, others are acute and require immediate intervention11 for optimal outcomes. See Table 2 for considerations on SS to add to patient education material for the most worrisome physical manifestations of these deficiencies, and Table 3 for common physical SS of nutrient deficiencies related to neurological conditions.
Guidelines from the American Society for Metabolic and Bariatric Surgery (ASMBS), American Association of Clinical Endocrinologists (AACE), and The Obesity Society (TOS) all recommend lifelong micronutrient supplementation at specific and evidence-based doses to protect the patients from nutrient deficiencies, along with serum marker screening and assessment of physical signs and symptoms.15,16 Studies continue to show that patients who adhere to these recommendations have significantly lower nutrient deficiency rates than those who take supplements, but not at the recommended prevention levels for a post-MBS patient, or patients who take no supplements at all.17
While lifelong annual screening of serum markers is indicated for all patients who undergo MBS, the reality of this occurring is often low—especially lacking is long-term follow up with a specialized bariatric center. In a 2019 single-center retrospective review (2001–2016) of 1,490 patients who underwent RYGB, it was determined that primary care providers were managing the care of the highest proportion of patients who underwent MBS within three years of their surgical date.18
In this same study, the authors found that the majority of patients did not have an assessment of basic nutrient screening markers. Five years after surgery, vitamin D, intact parathyroid hormone (iPTH), and vitamin B12 were not assessed in 82 percent, 85 percent, and 68 percent of patients, respectively. By 10 years postoperative, only 10 percent of patients available for data collection (35% of study population) had serum screening of vitamin B12.18 Considering this, it stands to reason that atypical nutrient screening markers, such as thiamin and copper, are not evaluated in a vast majority of patients long-term. This might potentially cause the HCP to miss any downward trends or low serum levels that might assist in the prevention of disastrous neurological complications to the patient.
This article aims to educate the HCP about the various nutrients that impact neurological health and that can lead to these devastating conditions. Although nutrient deficiencies are not the only reason for the development of neurological disorders after MBS, they are the most common cause.7,12 It is important to note that mechanical unloading of body weight and inflammatory mechanisms might also lead to neurological disorders, but those mechanisms will not be discussed in this article.14,19
Neurological Disorders Following MBS
Neurological disorders after MBS have an estimated incidence of up to 16 percent per year,14 although true rates are difficult to estimate because the majority of the body of literature are from case reports, with few retrospective studies in which micronutrient analysis are not consistent or uniform. Additionally, many of the studies are missing neuropsychological assessments and additional diagnostic measures (i.e., electrophysiology studies).7 There is a great need for large, long-term prospective studies to assess the true incidence and clinical outcomes of nutrition-related neurological disorders.2 Also, many nutrient-related complications might be missed due to lack of follow up to the bariatric group, unintentional HCP lack of awareness of the manifestations of these conditions, and unintended patient acceptance of certain signs and symptoms of these disorders.3
For example, many patients come to accept their body feeling “different” than it did before surgery and might not report classic signs and symptoms of nutrient deficiencies that lead to long-term neurological conditions. One illustration of this is unsteady gait, which is one of the most common initial symptoms of neurological disorders. While long-term studies on gait changes after surgery report an overall improvement, many patients describe feeling “off balance” in the early postoperative period as their body weight adjusts.20 This is likely due to a rapid mechanical unloading of body weight and while this might be the case initially, if the patient notices these same feelings further out from surgery, when this would not be expected, they might not think to report it to an HCP.
The most frequently reported neurological conditions after MBS are:
- Encephalopathy
- Polyradiculoneuropathy
- Optic neuropathy
- Myelopathy
- Peripheral and mononeuropathies
- Myopathy
Early postoperative neurological symptoms (typically presents within a few weeks to three months after surgery). Encephalopathy. One of the most acute neurological complications following MBS is encephalopathy and can present within the first few weeks to months after surgery. In post-RYGB patients who present with neurological complications, up to 40 percent of them will go on to develop encephalopathy, and a majority of them are found to be deficient in thiamin.21 See WE box on next page for more information.
Polyradiculoneuropathy. Another rapidly progressive manifestation of thiamin deficiency reported within the first six weeks after surgery is polyradiculoneuropathy, which resembles a longer-term disorder Guillain-Barré syndrome (GBS). Unlike GBS, patients with acute polyradiculoneuropathy have been found to have axonal degeneration and did not have an increase in cerebrospinal fluid (CSF) protein.13 Electromyography (EMG) can confirm diagnosis, which often shows axonal sensorimotor polyradiculoneuropathy. This tends to be more severe in the legs and is characterized by axonal degeneration without demyelinization.7
- Suggested treatment is parenteral thiamin supplementation of 100mg per day until symptoms resolve. This should be followed by longer-term higher oral doses for prevention; however, the adequate length of time has not been studied and HCPs will need to individualize their recommendations based on the particular case.7
In a review paper of 26 patients who developed critical neurological disorders after MBS, five (18.5%) were diagnosed with polyradiculoneuropathy. Four of the patients were diagnosed within three months postoperative, and one was diagnosed at two years postoperative.13 In the group of patients who presented acutely, all had low serum levels of vitamin B1. This was not attributed to frequent vomiting, but to severely rapid weight loss. Their condition did not resemble critical illness neuropathy; because there was no involvement in the phrenic nerves, they all reported pain in the feet or lower back with paresthesia (tingling, pricking, chilling, burning, or numbness) that began in the feet and ascended.
The next symptom reported was leg weakness, with an onset of hours to days after the initial symptoms began, and all became bedridden within a week of onset.
When it comes to suspected encephalopathies and acute polyradiculoneuropathy, the presumed thiamin deficiency should be emergently corrected. In most cases, this will require inpatient admission for parenteral nutrition supplementation. If patients report any gait changes, unusual sensations in their feet that ascend, have frequent and prolonged vomiting, ocular disturbances, or mental status changes especially in the first few months after surgery, a thiamin deficiency should be suspected, evaluated, and treated.
Mid-term postoperative neurological symptoms (typically presents 1 to 3 years after surgery). Optic Neuropathy. Typically associated with deficiencies in copper, and vitamins B12 or A, this disorder has been reported to occur between 18 months to 3 years postoperative.7,21,27 Symptoms tend to present with visual changes, including blurred vision, and can be accompanied by a blind spot in the middle of one’s vision.
In a case series of two female patients with a history of gastric bypass surgery, both presented with progressive optic neuropathy and low serum vitamin B12 levels.27 One patient was three years post-surgery and admitted to not taking any vitamin B12-containing supplements after surgery. The other patient was 1.5 years post-surgery and stated she stopped receiving B12 injections five months prior to the onset of symptoms.
The patients were started on vitamin B12 repletion therapy and despite normalization of serum B12 levels, their neurologic deficits continued. Upon further evaluation, severe copper deficiencies (serum copper levels were: <10mg/dL and 22mg/dL; normal range 70–155mg/dL) were discovered. Oral copper repletion was initiated (the authors do not offer the doses provided). One patient’s serum copper levels responded, and she experienced no further deterioration of symptoms, which included diminished peripheral vision, limited color vision, and difficulty in focusing on objects that were far away.27
The other patient’s serum copper levels continued to decline, and a GI workup revealed small intestinal bacterial overgrowth (SIBO) and intravenous (IV) copper was initiated, which improved her serum levels to the low-to-normal range. She reported no subjective improvement in her ophthalmologic complaints but experienced no further deterioration of symptoms, which included diminished peripheral vision and a severe reduction in in color vision.27
Long-term postoperative neurological symptoms (typically presents several years [5+] after surgery). Myelopathy. One of the most debilitating issues that can be caused by nutrient deficiencies following MBS is myelopathy.12 It is also one of the less frequently reported complications due to its long delay from surgery to diagnosis. The average time to presentation in the literature is nine years postoperative.12 Case reports involving copper deficiency that led to myelopathy after MBS have been seen as late as 24 years postoperative.7
Symptoms typically include the following:12
- Ataxic gait, spasticity with hyperreflexia, loss of vibratory sensations, and loss of pinprick and temperature sensation
This disorder is typically associated with vitamin B12 deficiency, but it can also be caused by deficiencies in copper, folate, and vitamin E.13 Treatment of myelopathies after MBS are dependent on the identified micronutrient deficiency. Despite repletion therapy, the majority of patients diagnosed with this condition remain disabled. For some patients, gait ataxia continued after diagnosis and repletion therapy for an additional three years, at which point the cases were no longer followed.7 One patient with myelopathy had improvement of symptoms following a surgical revision to shorten the bypassed intestine.13
Peripheral neuropathies. Peripheral, mono-, and polyneuropathies have been described in the literature. Neuropathies associated with MBS are likely due to a combination of nutrient deficiencies, not simply one isolated nutrient. Typical nutrient deficiencies related to peripheral neuropathies include: thiamin, vitamins E, B6 and B12, folate, and copper.21
Risk factors in the development of polyneuropathies include: rate and amount of weight loss, prolonged gastrointestinal symptoms, not attending nutrition appointments after surgery, low serum albumin and transferrin levels, and postoperative surgical complications that require hospitalization.14
Symptoms are usually described as the following:28
- Burning feet syndrome and distal loss of pinprick and temperature sensation
Along with assessing for and correcting micronutrient deficiencies, physical therapy has been shown to help improve outcomes for patients who develop peripheral neuropathies after MBS.28 Carpal tunnel syndrome, a form of mononeuropathy, is also a very common reported long-term symptom after MBS.12 Although rare, additional reported mononeuropathies include: ulnar, radial, peroneal, and lateral femoral cutaneous.21
Myopathy. Representing only two percent of neurological disorders reported in a group of 139 post-RYGB patients who presented with neurological disorders, it is typically associated with deficiencies of global protein, vitamin D, copper, or low blood levels of potassium.13 Additional nutrients proposed to lead to myopathy include: thiamin, vitamin E, selenium, calcium, phosphate, and magnesium.12
Symptoms may include the following:7
- Weakness with diffuse pain

Nutrient Deficiencies
It is well established that obesity is a pre-existing condition for micronutrient deficiencies. Many patients who seek surgery have one or more micronutrient deficiency that can impact neurological health.5,15,29 After surgery, patients are often at even greater risk of developing these deficiencies due to: prolonged vomiting, inadequate micronutrient supplementation, malabsorption, bacterial overgrowth, and rapidity of weight loss.7,30
Nutrient deficiencies involved in the pathway of neurological conditions include:
- Thiamin (vitamin B1)
- Vitamin B12
- Vitamin B2
- Vitamin B6
- Folate
- Vitamin A
- Vitamin D
- Vitamin E
- Copper
The majority of data that describe how nutrient deficiencies have led to neurological disorders after MBS come primarily from case reports and case studies, with minimal large prospective trials and retrospective studies.7,10,14 Since the body of literature is focused on thiamin, vitamin B12, and copper deficiencies we will go into detail below regarding those specific nutrients and the role they play in neurological conditions. We will also review screening methods, prevention dose guidelines, and repletion strategies. See Table 4 for pathways involved in the deficiencies of other nutrients mentioned above, and see the ASMBS 2016 micronutrient update guidelines for prevention and repletion doses of the additional micronutrients that are not covered in this article.15
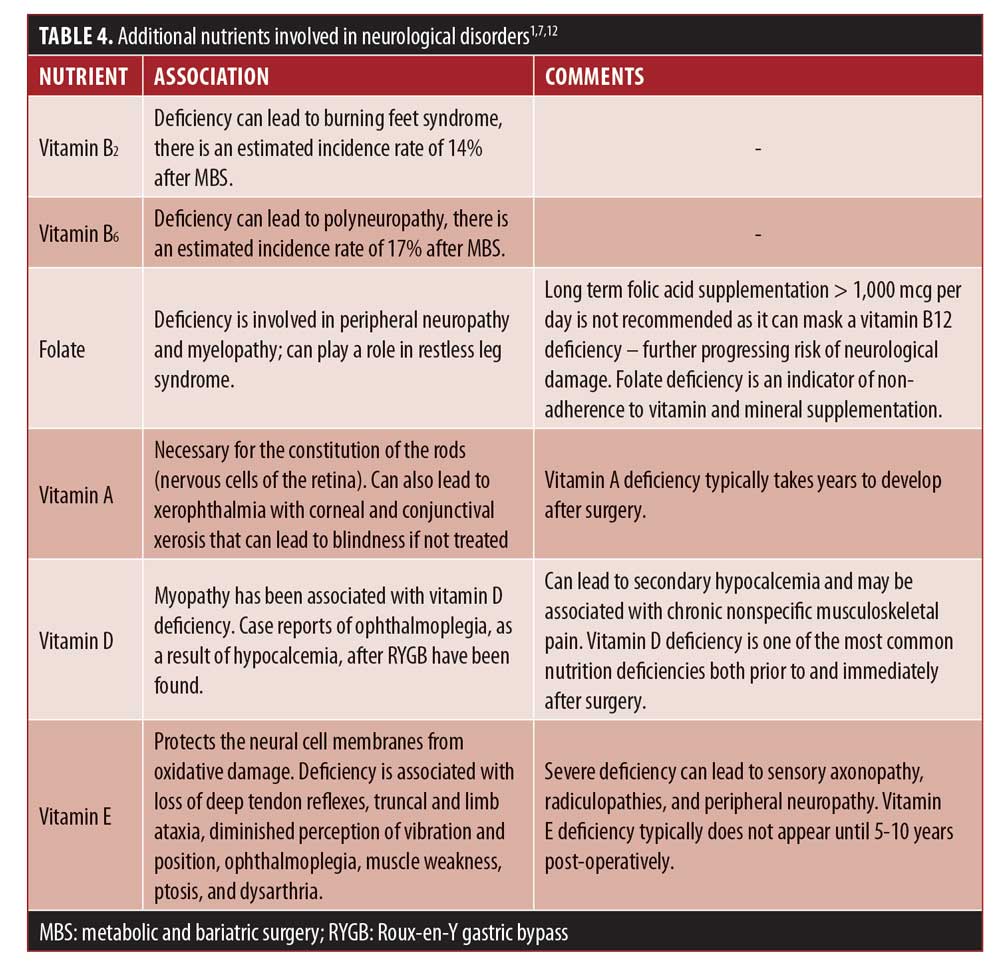
Thiamin. A water-soluble vitamin essential for energy production and myelin formation in nerve cells, which cannot be produced by the body and must be exogenously provided.7,11
Risk of deficiency. The body stores approximately 25 to 30mg of thiamin, which can be depleted within 2 to 3 weeks if intake does not meet needs.31 The prevalence of thiamin deficiency prior to surgery has been reported to be as high as one out of three patients, making preoperative screening especially critical.15 Thiamin deficiency changes mitochondrial function by inhibiting oxidative metabolism and diminishes vitamin B1-dependent enzymes, which results in selective neuronal cell death.32
The awareness of thiamin deficiency is critical for HCPs and patients because it is significant in the development of acute disorders, such as WE, which can lead to the chronic encephalopathy known as Korsakoff’s syndrome, a permanent impairment of short-term memory.33 Typically, thiamin deficiency has been attributed to rapid weight loss, vomiting, and inadequate supplementation.28 Deficiency can still present despite supplementation if the patient experiences persistent and intractable vomiting.
Typical time of onset. Thiamin deficiency is one of the only known acute micronutrient deficiencies after MBS, as it can present as early as six weeks following surgery. Typical time to presentation is 8 to 15 weeks postoperative.34 There is also a long-term risk of deficiency, particularly if the patient begins consuming large quantities of alcohol or carbohydrates, or develops anorexia.6,35
Progression of disorder and anticipated outcome. Early symptoms of thiamin deficiency include gait ataxia, polyneuropathy, ophthalmoparesis, nystagmus, and confusion.33 Recovery is often dependent on how early the symptoms are recognized. If discovered early, and appropriate nutrition therapy is initiated, certain symptoms (rapid eye movement) are expected to resolve within a few days, while others might take 3 to 6 months (mental status) to show improvement.36 In mild deficiency cases, SS might include malaise, irritability, and confusion.37 In severe cases, especially if the deficiency is not caught early, patients could be left with permanent deficits.37
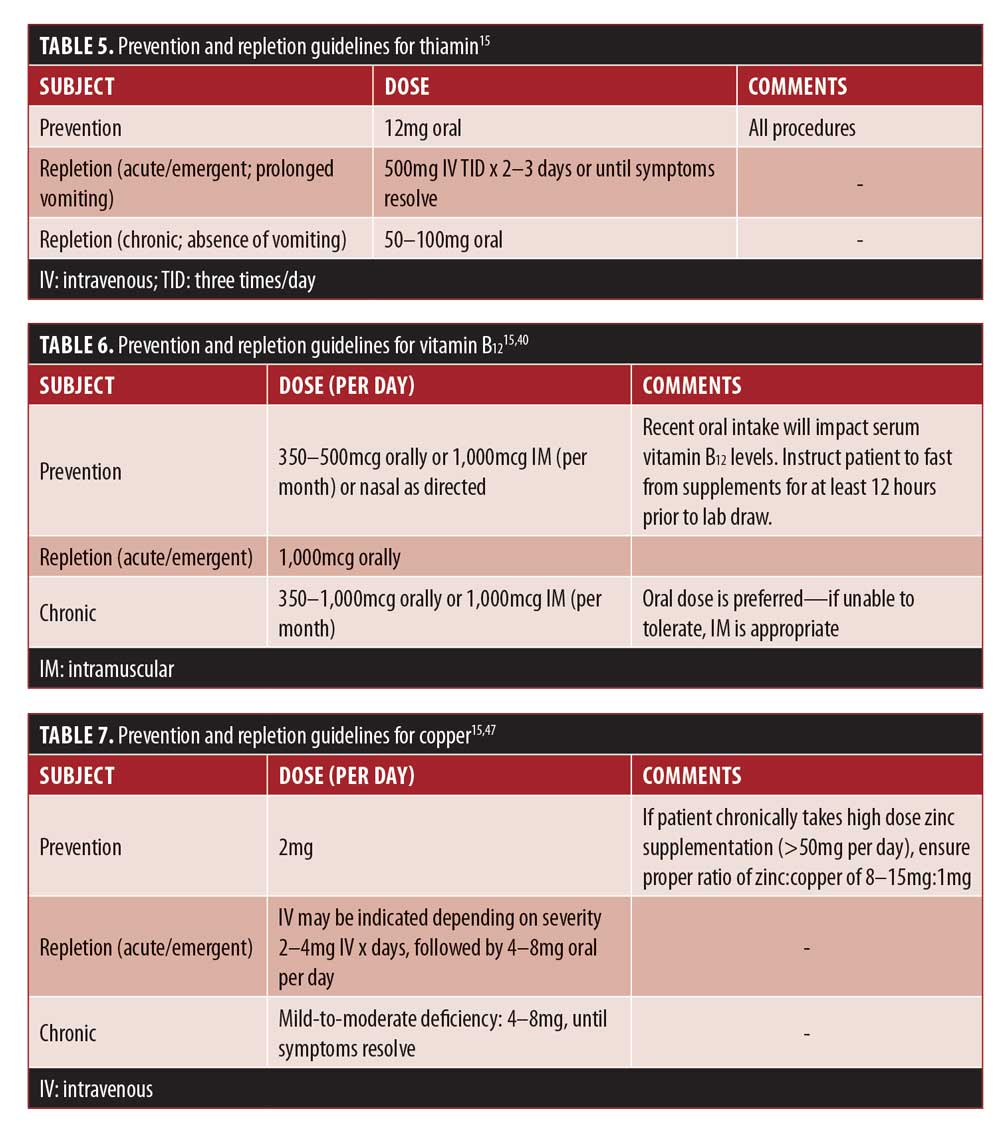
Diagnosing deficiency. One of the challenges with diagnostic testing for thiamin lies in the methods of collection and rapidity of results.6 Around 90 percent of the thiamin present in whole blood is found in thiamin diphosphate (TDP), which is considered to be the most sensitive measure of status. Results of <70nmol/L are indicative of a deficiency.35 However, it typically takes 2 to 3 days to analyze the sample.38 Additionally, recent supplement intake can interfere with testing results. Patients should be instructed to fast from supplements for 12 to 14 hours prior to collection.35 See Table 5 for more information.
Vitamin B12. A water soluble vitamin that plays a role in myelin synthesis and in methylation of ribonucleic acid (RNA).7
Risk of deficiency. Deficiency of vitamin B12 can be attributed to inadequate supplemental intake, impaired hydrolysis of dietary protein, and reduced or nearly absent (in the case of the SG) intrinsic factor.28 The absorption of vitamin B12 is dependent on its ability to bind to intrinsic factor, which is produced by the parietal cells in the antrum of the stomach. It is primarily absorbed actively in the ileum as a bound complex; however, an estimated one percent can be absorbed via passive diffusion, which is likely how the majority of post-MBS patients are able to absorb their needs.39
Typical time of onset. The body stores a large amount of vitamin B12 in the liver; for this reason, low levels are not expected to present until years after surgery. The prevalence of B12 deficiency prior to surgery is relatively low and is estimated to be 2 to 18 percent, with higher rates (30%) for patients taking proton-pump inhibitors.15
Progression of disorder and anticipated outcome. Postoperative deficiency studies indicate that vitamin B12 can efficiently be corrected with high enough doses of daily oral vitamin B12. The most recent 2016 ASMBS guidelines indicate 1,000mcg of oral B12 daily is effective at correcting deficiency.15 In a more recent systematic review, the authors suggest a much lower dose of 350mcg per day as being adequate to correct deficiency.40 The clinician will likely need to assess for recent vitamin B12 intake and individualize the repletion treatment plan based on what the patient had (or had not) been taking prior to the identified deficiency.
Alternatively, 500mcg weekly via nasal route or 1,000mcg intramuscularly (IM) monthly to 1,000 to 3,000mcg IM every 6 to 12 months have proven sufficient to replete B12 stores if adequacy cannot be achieved using oral administration.40 Of interest, the recommendation of sublingual administration route is not based on validated studies, although this is a common recommendation.41
The goal of repletion therapy is to improve symptoms, serum markers (preferably both serum vitamin B12 and methylmalonic acid [MMA]), and repletion of cobalamin stores. In the case of neurological impact related to a vitamin B12 deficiency it might take several months of repletion therapy to improve the clinical symptoms.41,42
Diagnosing deficiency. Serum vitamin B12 is a typical marker used to evaluate deficiency; however, this might miss 30 to 50 percent of vitamin B12 deficiencies, which is why serum MMA is the preferred method for assessment.15
Neurological symptoms present in vitamin B12 deficiency include paresthesias, weakness, decreased reflexes, ataxia, vibratory sense loss, dementia, psychosis, and altered mood.43 See Table 6 for more information.
Copper. A trace mineral that is an essential cofactor in multiple enzymatic reactions critical to the neurological system.44
Risk of deficiency. Relatively rare, but likely underdiagnosed, copper deficiency can present after malabsorptive procedures.44 Absorption occurs in the stomach and duodenum. The average adult stores approximately 100mg of copper, primarily in the liver, brain, heart, and kidneys.31
Typical time of onset. Most case reports of copper deficiency are from patients who have had surgery more than 10 years prior to onset of deficiency related symptoms.44
Progression of disorder and anticipated outcome. Symptoms of copper deficiency often mimic vitamin B12 deficiency, including peripheral neuropathy, optic neuropathy, central nervous system demyelination, myopathy, and myelopathy with a spastic ataxic gait.7 The neurological conditions associated with copper deficiency can be profound and are frequently irreversible. Early diagnosis, awareness of deficiency risk, and proper screening measures are important in the outcome of each case.44
Unfortunately, due to the delay of onset from surgery to deficiency, case reports suggest that by the time the patient presents with symptoms, they are typically severely depleted. Hematologic indices are shown to rapidly improve with IV copper and high dose oral repletion; however, the neurologic deficits and symptoms usually show only slight improvement over a several month time period.44
The response to copper repletion therapy reported in the literature is variable and likely dependent on the degree and duration of depletion. Serum marker trends will likely show improvement within a few weeks of initiating therapy, but subjective improvement in symptoms are more challenging to assess, and often patients are left with permanent impairments, especially in gait abnormalities.44,45
Diagnosing deficiency. Prevention of copper deficiency suggests an oral dose of 2mg per day,15 with careful attention paid to patients who take high-dose zinc supplementation for long periods of time. The intestinal metal binding protein metallothionein has a greater affinity for copper than zinc or other metals. When high doses of zinc are consumed, copper can become trapped in the enterocyte, sloughed off and eliminated within the intestine, thereby decreasing its absorption.15
Laboratory assessment shows that patients with copper deficiency tend to exhibit hypochromic anemia and neutropenia with normal iron studies and occasionally elevated ferritin.46 Depleted serum copper (normal range 70–145mcg/dL) and ceruloplasmin (normal range 27–37mg/dL) are also noted but rarely assessed.47 Note, they are also acute phase reactants and will be falsely elevated in the presence of inflammation, age, anemia, and certain medications.15 See Table 7 for more information.
Conclusion
As the number of MBS procedures continues to increase, patients and clinicians need to be aware of the nutritional complications that can present both in the first few weeks after surgery as well as decades later. Learning how to recognize, assess, and treat these often life-altering deficiencies is paramount to a patient’s health. Emergent symptoms of nutrition deficiencies should play a major role in nutrition education.
The patients need to understand that confusion, memory issues, strange ocular movement, vision impairment, weakness, odd sensations particularly in the limbs, and quick-onset gait ataxia are not expected to occur and to immediately contact their HCP should they experience any of these changes. If patients do present with these symptoms testing for thiamin, folate, vitamins B12, A, E, D, zinc, and copper is warranted as well as assessing for weight loss, recent GI issues, and micronutrient supplementation intake.
References
- Shils M, Olson J, Shike M, et al. Modern Nutrition in Health and Disease 9th edition. Baltimore, MD: Lippincott Williams and Wilkins, 1999.
- Punchai S, Hanipah Z, Meister K, et al. Neurologic manifestations of vitamin B deficiency after bariatric surgery. Obes Surg. 2017;27:2079–2082.
- Chang H, Yang P, Han T, et al. Wernicke encephalopathy concurrent with polyradiculoneuropathy in a young man after bariatric surgery. Medicine. 2019;98(10):e14808.
- Medical dictionary online. https://www.online-medical-dictionary.org/. Accessed August 3, 2020.
- Frantz DJ. Neurological complications of bariatric surgery: involvement of central, peripheral, and enteric nervous system. Curr Gastroenterol Rep. 2012;14:367–372.
- Algahtani H, Khan A, Khan M, et al. Neurological complications of bariatric surgery. Neurosciences. 2016;21(3):241–245.
- Landais A. Neurological complications of bariatric surgery. Obes Surg. 2014;24:1800–1807.
- Oudman E, Wijnia J, van Dam M, et al. Preventing Wernicke encephalopathy after bariatric surgery. Obes Surg. 2018;28:2060–2068.
- Via M, Mechanick J. Nutritional and micronutrient care of bariatric surgery patients: current evidence update. Curr Obes Rep. 2017;6:286–296.
- Abarbanel J, Berginer V, Osimani A, et al. Neurological complications after gastric restrictions after gastric restriction surgery for morbid obesity. Neurology. 1987;37:196–200.
- Tabbara M, Carandina S, Bossi M, et al. Rare neurological complications after sleeve gastrectomy. Obes Surg. 2016;26:2843–2848.
- Becker D, Balcer L, Galetta S. The neurological complications of nutritional deficiency following bariatric surgery: review article. J Obes. 2012;2012:608534.
- Juhasz-Pocsine K, Rudnicki S, Archer R, et al. Neurologic complications of gastric bypass surgery for morbid obesity. Neurology. 2007;68(21):1843–1850.
- Thaisetthawatkul P, Collazo-Clavell M, Sarr M, et al. A controlled study of peripheral neuropathy after bariatric surgery. Neurology. 2004;63(8):1462–1470.
- Parrott J, Frank L, Rabena R, et al. American Society for Metabolic and Bariatric Surgery Integrated Health Nutritional Guidelines for the Surgical Weight Loss Patient 2016 Update: Micronutrients. Surg Obes Relat Dis. 2016;12:955–959.
- Mechanick J, Apovian C, Brethaur S, et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures – 2019 update: cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic & Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Surg Obes Relat Dis. 2020;16(2):175–247.
- Homan J, Schijns W, Aarts E, et al. An optimized multivitamin supplement lowers the number of vitamin and mineral deficiencies three years after roux-en-y gastric bypass: a cohort study. Surg Obes Relat Dis. 2016;12:659–667.
- Fox W, Borgert A, Rasmussen C, et al. Long-term micronutrient surveillance after gastric bypass surgery in an integrated healthcare system. Surg Obes Relat Dis. 2019;15(3):389–395.
- Alves L, Goncalves R, Cordeiro G, et al. Beriberi after bariatric surgery: not an unusual complication. Report of two cases and literature review. Arq Bras Endocrinol Metabol. 2006;50:
564–568. - Vincent H, Ben-David K, Conrad B, et al. Rapid changes in gait, musculoskeletal pain, and quality of life after bariatric surgery. Surg Obes Relat Dis. 2012;8(3):346–354.
- Koffman B, Greenfield L, Ali I, et al. Neurologic complications after surgery for obesity. Muscle Nerve. 2006;33(2):166–176.
- Aasheim E. Wernicke encephalopathy after bariatric surgery: a systematic review. Ann Surg. 2008;248(5):714–720.
- Sinha S, Kataria A, Prakash B, et al. Wernicke encephalopathy – clinical pearls. Mayo Clin Pract. 2019;94(6):1065–1072.
- Armstrong-Javors A, Pratt J, Kharasch S. Wernicke encephalopathy in adolescents after bariatric surgery: case report and review. Pediatrics. 2016;138(6):e20161039.
- Sechi G, Serra A. Wernicke’s encephalopathy: new clinical settings and recent advances in diagnosis and management. Lancet Neurol. 2007;6:442–455.
- Sechi G. Prognosis and therapy of wernicke’s encephalopathy after obesity surgery. Am J Gastroent. 2008;103(12):3219.
- Pineles S, Wilson C, Balcer L, et al. Combined optic neuropathy and myelopathy secondary to copper deficiency. Surv Ophthalmol. 2010;55(4):386–392.
- Berger J. The neurological complications of bariatric surgery. Arch Neurol. 2004;61(8):1185–1189.
- Folope V, Coeffier M, Déchelotte P. Nutritional deficiencies associated with bariatric surgery. Gastroenterol Clin Biol. 2007;31:369–377.
- Sahin S, Florentina Ates M, Cinar N, et al. A new etiology for variant of guillain-barré syndrome: bariatric surgery. Eur Res J. 2019;5(6):1024–1027.
- Mahan L, Escott-Stump S. Krause’s Food, Nutrition, and Diet Therapy 10th edition. Philadelphia, PA: W.B. Saunders Company, 2000.
- Ke Z, DeGiorgio L, Volpe B, et al. Reversal of thiamine deficiency-induced neurodegeneration. J Neuro Exp Neuro. 2003;62(2):195–207.
- Dreyfus P, Victor M. Effects of thiamine deficiency on the central nervous system. Am J Clin Nut. 1961;9:414–425.
- Sola E, Morillas C, Garzon S, et al. Rapid onset of Wernicke’s encephalopathy following gastric restrictive surgery. Obes Surg. 2003;13(4):661–662.
- Mayo Clinic Laboratories. Test ID: TDP. Clinical & Interpretive. https://www.mayocliniclabs.com/test-catalog/Clinical+and+Interpretive/42356 . Accessed August 3, 2020.
- Koike H, Misu K, Hattori N, et al. Postgastrectomy polyneuropathy with thiamine deficiency. J Neurol Neuros Psy. 2001;71(3):357–362.
- Patrini C, Griziotti A, Ricciardi L. Obese individuals as thiamin storers. Int J of Obesity. 2004;28:920–924.
- Moore C, Sherman V. Effectiveness of B vitamin supplementation following bariatric surgery: rapid increases of serum vitamin B12. Obes Surg. 2015;25:694–699.
- Mahawar K, Reid, A, Graham Y, et al. Oral vitamin B12 supplementation after Roux-en-Y gastric bypass: a systematic review. Obes Surg. 2018;28:1916–1923.
- Smelt H, Pouwels S, Smulders J. Different supplementation regimes to treat perioperative vitamin B12 deficiencies in bariatric surgery: a systematic review. Obes Surg. 2017;27:
254–262. - Chan L, Mike L. The science and practice of micronutrient supplementations in nutritional anemia: an evidence-based review. J Par Ent Nut. 2014;38(6):656–672.
- NIH Office of Dietary Supplements. Office of Dietary Supplements – Vitamin B12. https://ods.od.nih.gov/factsheets/VitaminB12-HealthProfessional/ . Accessed July 30, 2020.
- Healton E, Savage D, Brust J, et al. Neurologic aspects of cobalamin deficiency. Medicine. 1991;70(4):229–245.
- Griffith D, Liff D, Ziegler T, et al. Acquired copper deficiency: a potentially serious and preventable complication following gastric bypass surgery. Obesity. 2009;17(4):827–831.
- Kumar N, Ahlskog JE, Gross JB. Acquired hypocupremia after gastric surgery. Clin Gastroenterol Hepatol. 2004;30:446–450.
- Shils M, Shike M, Ross A, et al. Modern Nutrition in Health and Disease. Vol 10th edition. Baltimore, MD: Lippincott Williams and Wilkins, 2006.
- Cummings S, Isom, K. WM DPG AND Pocket Guide to Bariatric Surgery Edition 2: Appendix D & E.
Category: Past Articles, Review



