Robotic Single-Anastomosis Duodenoileal (SADI) Bypass After Sleeve Gastrectomy for Insufficient Weight Loss

by Varun Jain, MBBS, MS, and Enrique F. Elli, MD, FACS, FASMBS
Dr. Elli is Chair of Advanced Gastrointestinal and Bariatric Surgery, Mayo Clinic Florida Department of Surgery, Mayo Clinic in Jacksonville, Florida. Dr. Jain is a Fellow (Advanced Gastrointestinal/ Bariatric Surgery), Mayo Clinic Florida Department of Surgery, Mayo Clinic in Jacksonville, Florida.
Funding: No funding was provided for this article.
Disclosures: The authors have no conflicts of interest relevant to this article.
Bariatric Times. 2022;19(7):10–13.
Abstract
Bariatric surgery numbers have continued to increase with the increasing prevalence of obesity. Surgical options have continued to evolve, and currently, sleeve gastrectomy is the most common procedure performed in the United States, constituting around 60 percent of all primary bariatric operations. As long-term data emerges, weight regain has been identified as one of the problems warranting a surgical revision. Revisional bariatric surgery is not only more technically challenging, but the decision process regarding appropriate procedure selection is also of utmost importance. With the increasing number of revisional procedures being performed, the bariatric surgeon should be familiar with the various options available. A single-anastomosis duodenoileal bypass with sleeve (SADI-S) is proving to be an effective option, specifically for tackling weight regain. The robotic surgical platform offers several advantages for primary bariatric procedures, but even more so for revisional procedures that typically involve bowel anastomoses. We have reviewed the SADI-S procedure as a revisional option after prior sleeve gastrectomy using the robotic surgical platform and describe our approach.
Keywords: Bariatric surgery; robotic bariatric surgery; bariatric revision; SADI; single anastomosis DS; weight regain
The concept of biliopancreatic diversion (BPD) was first described by Scopinaro et al1 in 1979. He originally described an open technique involving a distal gastrectomy leaving a 200 to 500cc capacity ad hoc stomach and a Roux-en-Y reconstruction with anastomosis 50cm proximal to the ileocecal junction.1 In 1988, Hess and Hess2 performed a hybrid procedure combining the Scopinaro BPD and DeMeester’s duodenal switch (DS). This was performed as an open procedure, involving preservation of the pylorus and creation of a gastric tube with division of the duodenum and a duodenojejunostomy for reconstruction. There were more reported major complications and mortality in patients with a body mass index (BMI) greater than 60kg/m2, along with additional challenge of laparoscopic surgery in this cohort.3 As a result, Ren et al4 proposed a two-stage procedure in which a sleeve gastrectomy was performed as the initial step, followed by a second stage biliopancreatic diversion.3,4 The interesting observation of significant percent excess weight loss (%EWL) after this first-stage sleeve gastrectomy led to its adoption as an independent primary bariatric procedure.
According to a review of bariatric procedures worldwide from 2011, an estimated 340,000 procedures were performed annually, with the most common procedure being Roux-en-Y gastric bypass (RYGB) at 46.6 percent, and sleeve gastrectomy (SG) being the second most common at 27.8 percent.5 However, over the past several years, SG has become the preferred procedure for most, constituting almost 60 percent of all primary bariatric procedures in the United States (US) as per American Society for Metabolic and Bariatric Surgery (ASMBS) estimates in 2019.6
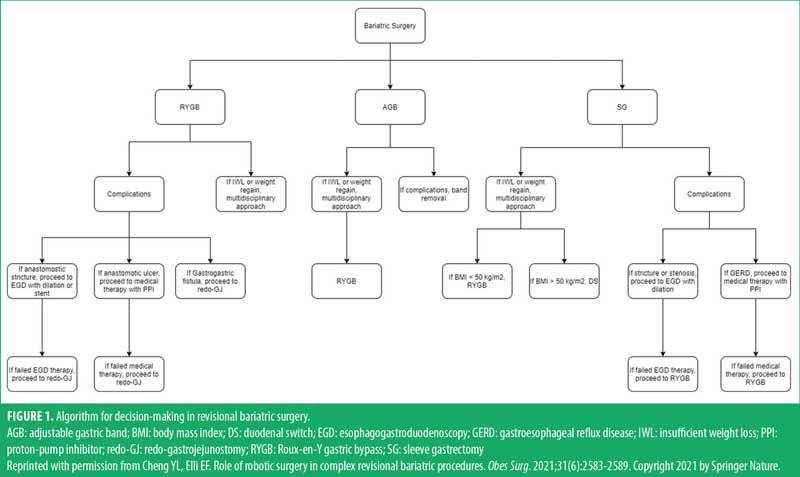
Long-term data on outcomes after SG are still evolving. There are several reports that have shown lower %EWL, higher weight regain, higher incidence of reflux, and lower metabolic comorbidity resolution after SG, compared to other metabolic procedures, such as gastric bypass.7–9 As a result, there has been an increasing number of conversions after SG to other procedures, such as a RYGB, repeat/revision SG, DS, or single-anastomosis duodenoileal bypass (SADI),10–12 and the rate of conversion seems to increase cumulatively to around 12 percent at 10 years.13
SADI with sleeve (SADI-S) (also known as one-anastomosis duodenal switch [OADS]) is a novel technique initially described by Sánchez- Pernaute et al14 in 2007.This is primarily based on the principal of biliopancreatic diversion, which aims to add an additional metabolic and malabsorptive component to SG, which is primarily restrictive, but also has inherent neurohormonal mechanisms contributing to weight loss, both of which might regress over time.14 While this can be performed as a primary bariatric procedure with an excellent safety profile and weight loss and metabolic results, it is also one of the potential revision procedures after a failed SG.15,16 In addition to the metabolic advantages of conversion to SADI, one additional factor that could potentially contribute to the wider adoption of complex revisional bariatric procedures in general is the growing experience with the surgical robotic system and the shorter learning curve, especially for more complex tasks, such as suturing, although there is no reliable clinical data to support this. In a retrospective review of robotic revisional bariatric surgery at our institution, we found it to be safe, with a low rate of major adverse events.11 There are several other published reports that have reported similar favorable outcomes for robotic revisional bariatric procedures.17,18 For primary procedures, the elimination of one anastomosis with a SADI-S had the hypothesized, as well as reported, benefit of reducing operative time, as well as surgery- and anastomosis-related complications.15 We feel this benefit could translate even to revision procedures, although data on the actual benefit for revision procedures is lacking.
Key Preoperative Steps
Patient selection. We established a stepwise algorithm when approaching patients for revisional bariatric surgery that takes into account their presenting symptoms, present BMI, and overall fitness for surgery.
In patients who present after prior SG with either inadequate weight loss or weight regain, we have a multidisciplinary discussion to determine the appropriate revisional procedure. Other indications for conversion after SG included gastroesophageal reflux disease (GERD) refractory to medical therapy and anatomic complications, such as sleeve angulation, strictures, or migration into the mediastinum.
In patients with a BMI of 50kg/m2 or greater, our preference is for a standard two-anastomosis DS or SADI-S, as DS has been shown to provide superior metabolic comorbidity resolution, compared to gastric bypass.19–21 Even though quality evidence is lacking, that has been our anecdotal experience as well. Other factors considered in determining the suitability of SADI as a revision procedure after prior SG are medical comorbidities, presence of a hiatal hernia, sleeve anatomy, and duration since initial procedure. The presence of recalcitrant reflux symptoms or esophagitis warrants further investigation. Objective evidence of significant GERD usually precludes a DS/SADI, and in those patients, our revisional procedure of choice is a RYGB.
Preoperative workup. All candidates being considered for revisional bariatric surgery are seen by a multidisciplinary bariatric team not only to undergo appropriate preoperative investigations, but also for specific education regarding the options, outcomes, and lifestyle modifications after revision procedures.
All patients undergo an upper gastrointestinal (GI) series with contrast and an upper endoscopy for anatomic evaluation, including signs of acid reflux, such as esophageal erosions/ulcerations, Barrett’s esophagus, or other precluding esophagogastric lesions. Twenty-four hour pH monitoring and manometry is performed selectively for evaluation of reflux/dysphagia symptoms or concerning endoscopic findings.
All patients are seen in a preoperative medical/anesthesia evaluation clinic for risk factor assessment and optimization. Cardiopulmonary testing is individualized.
Operating room setup and patient positioning. Patients are positioned on the operating table supine with arms out and legs split. Straps or tape is used to secure the knees, and additional support is provided by footboards, especially when in reverse Trendelenburg position. We do not routinely place foley catheters, but they may be individualized depending on anticipated complexity and duration of the operation. Bony prominences over the arms, as well as the heels, are appropriately padded, and an upper body patient warming system is placed. All patients have sequential compression devices on bilateral legs and receive thromboprophylaxis and preoperative antibiotics per protocol.
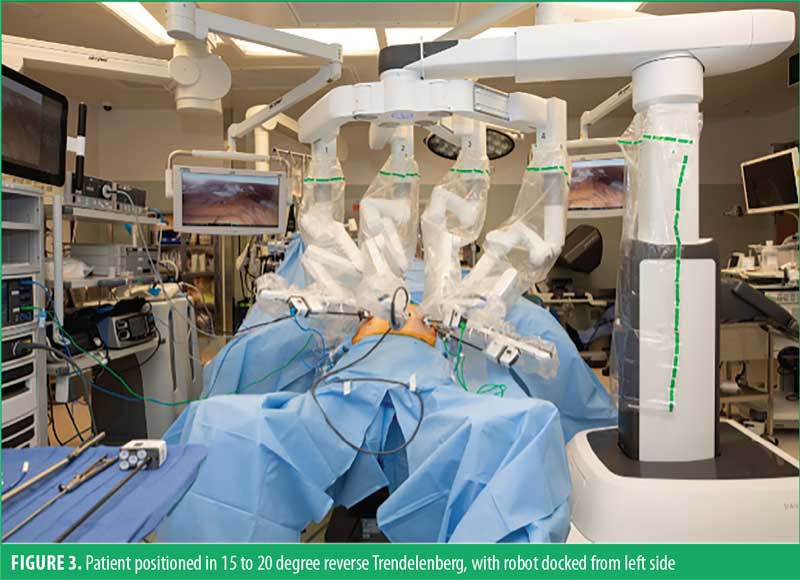
We perform the procedure robotically using the DaVinci Xi robot (Intuitive Surgical, Inc.; Sunnyvale, CA, United States [US]). We dock from the patient’s left side, but it may be performed from any direction. A side dock approach allows more space and mobility at the head of the bed for the anesthesiologist and facilitates interventions, such as passage of tubes, bougies, and intraoperative endoscopy. The scrub technician is positioned on the patient’s right side by the foot end and all wires, cords, and tubing are passed off to the patient’s right side.
Key Operative Steps
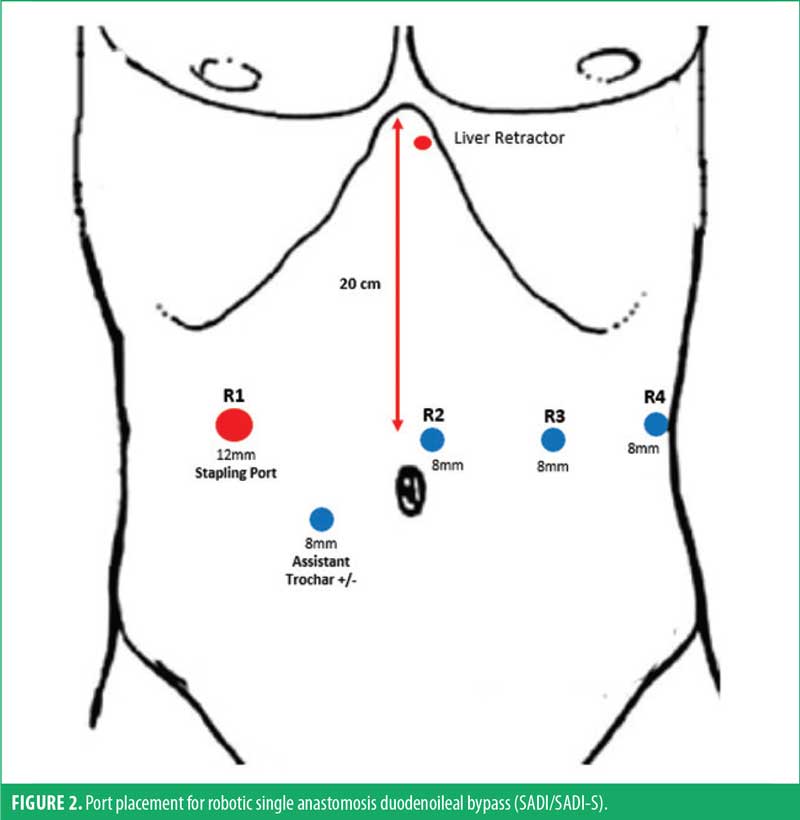
Entry and port placement. We use a 5mm optical viewing trocar for initial access in the left mid-abdomen (at the R3 site in Figure 1). This is performed with clear visualization of abdominal wall layers and with precise control. We have found this to be an expeditious yet safe technique for intraperitoneal access. A carbon dioxide insufflator is then connected to displace omentum or any other viscera and establish pneumoperitoneum to 15mmHg. We routinely perform a diagnostic laparoscopy focused on evaluation of intra-abdominal adhesions, small bowel mobility, or other obvious anatomic or structural abnormalities.
A Nathanson self-retaining liver retractor (Cook Medical; Bloomington, IN, US) is placed via a 5mm subxiphoid incision under direct visualization. Additional 8mm robotic trocars are then placed under direct visualization in the right abdomen, periumbilical area, and left lateral abdomen. Finally, the initial 5mm optical viewing trocar is switched out for a 12mm trocar (Figure 1). The 12mm port serves as the stapling port as it allows an in-line approach for duodenal transection. We do not routinely use any additional assistant trocars. Sutures and sponges are preplaced in the abdomen as needed, prior to docking the robot from the patient’s left side (Figure 2).
Surgical technique. After initial diagnostic laparoscopy and lysis of adhesions as necessary, we begin by identifying the terminal ileum and ileocecal junction. The small bowel is traced retrograde from the ileocecal junction for a length of 250cm using a tape measure, with the bowel on minimal stretch. This is marked with a 3-0 monofilament delayed absorbable suture (PDS™, Ethicon; Raritan, NJ, US). The robot is then docked from the patient’s left side, as detailed above. We typically begin with a fenestrated bipolar forceps in R1, camera in R2, monopolar hook with electrocautery in R3, and Cadiere forceps in R4.
We begin with attention to the upper abdomen and evaluate the stomach anatomy, which includes sleeve orientation, sleeve caliber, perigastric/periduodenal adhesions, or presence of a hiatal hernia. We do not routinely undertake a hiatal dissection unless there is preoperative evidence or obvious operative findings to suggest a hiatal hernia. If one is noted, appropriate hiatal and mediastinal dissection is completed in order to obtain at least 3cm intra-abdominal esophageal length, followed by hiatal closure with interrupted permanent braided suture (Ethibond Suture 0, Ethicon; Raritan, NJ, US). In cases where the prior gastric sleeve appears dilated on preoperative imaging, we occasionally will perform a sleeve revision using a 50Fr bougie for appropriate caliber. Similarly, for primary DS procedures, we use the same caliber bougie.
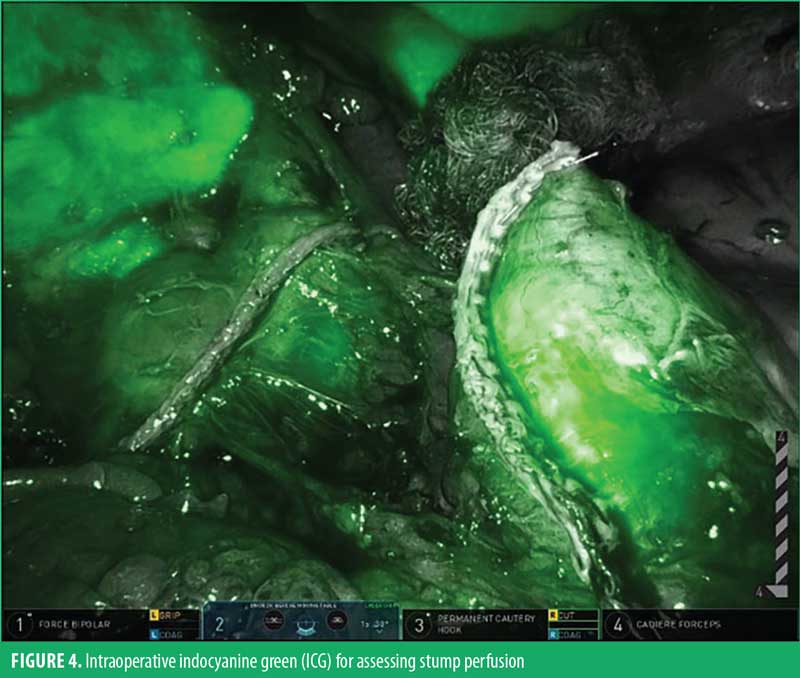
Next, the pylorus is identified based on visual inspection, as well as occasionally using the anterior pyloric vein as a landmark. Using electrocautery the pylorus, as well as the first and second portions of the duodenum, are mobilized, remaining close to the viscera. The key portion of the procedure is creation of the retroduodenal window using a combination of blunt and sharp dissection with appropriate use of electrocautery. It is important to try and achieve this without dividing any of the major vessels (i.e, right gastric artery or right gastroepiploic artery). We also make an effort to preserve any prominent periduodenal vessels by identifying a fine dissection plane between them and the bowel wall. We use intraoperative fluorescence imaging with indocyanine green (ICG) dye for evaluation of tissue vascularity. We have found this to be especially useful in cases where conduit mobilization is challenging, which occasionally requires division of the right gastric artery.
Once the retroduodenal window is created, duodenal transection is performed around the D1/D2 junction, leaving a 2 to 4cm cuff of duodenum using a 60mm blue load robotic stapler (Sureform 60 with SmartFire, Intuitive Surgical, Inc.; Sunnyvale, CA, US) without tissue reenforcement perpendicular to the long axis. At this point, the perfusion of both ends of the transected duodenum is evaluated using ICG and integrated fluorescence imaging (Figure 3).
The omentum is divided longitudinally to facilitate the reach of the jejunal loop for a tension-free anastomosis. The marked jejunal loop is then brought up to the proximal duodenal staple line in an antecolic fashion and a sutured end to side duodenojejunostomy is performed in two layers. A posterior continuous layer is first performed using 3-0 monofilament delayed absorbable suture, followed by duodenotomy and enterotomy using hook electrocautery for a length of 2cm. We then perform the inner layer of the anastomosis, incorporating mucosa in a running fashion beginning posteriorly using 3-0 monofilament delayed absorbable suture, which is then continued forward to complete the anterior layer. A second outer layer of Lembert seromuscular sutures is then placed, once again using a continuous layer of 3-0 monofilament delayed absorbable suture. Two additional sutures are then placed between the biliopancreatic limb and the gastric antrum to introduce an angulation that is intended to prevent reflux proximally into the anastomosis.
A thorough evaluation is performed for hemostasis, following which the robot is undocked and an intraoperative upper endoscopy is performed. The bowel is clamped and an upper endoscopic evaluation is performed by inspecting and passing the scope through the anastomosis to confirm both hemostasis as well as patency. We then close the 12mm robotic trocar site using 0 Vicryl suture in a figure-eight fashion under direct visualization, using a Carter-Thomason fascial closure needle (Cooper Surgical; Trumbull, CT, US). The liver retractor is also removed under direct visualization, the abdomen is desufflated, and all port site skin incisions are closed using 4-0 absorbable monofilament suture (Monocryl®, Ethicon; Raritan, NJ, US). Typical operative time is around 90 to 100 minutes for this procedure.
Postoperative Recovery and Follow-up
We do not routinely perform a postoperative contrast swallow/small bowel follow-up evaluation. Patients are usually initiated on postbariatric, sugar-free, clear liquids right after surgery. There is a protocol for gradual advancement as tolerated. All patients postoperatively receive bilateral leg sequential compression devices as well as injectable subcutaneous venous thromboembolism (VTE) prophylaxis and ambulation. Most patients are typically discharged on postoperative day 1 or 2, after which they follow an outpatient diet advancement plan. The initial postoperative visit in the surgery clinic is three weeks after discharge. Further outpatient follow-up occurs through the multidisciplinary bariatric clinic, consisting of a medical bariatrician, advanced practice providers, and nutritionist. With DS, SADI-S, and other biliopancreatic diversion procedures, there is an enhanced follow-up plan, with more frequent nutritional parameters, multivitamin and protein goal assessments, and dietary counseling, compared to the more commonly performed SG or RYGB. This is done in an effort to limit nutritional deficiencies, such as vitamins and protein-calorie malnutrition, that typically occur more frequently after these procedures.
Discussion
Several studies have demonstrated the overall health and life expectancy benefits of bariatric surgery.22–24 Weight regain after initial rapid weight loss after these procedures remains a concern. A prospective study by Adams et al25 reported roughly 20 to 25 percent of the initial weight lost at two years following RYGB was regained over the subsequent 10 years. SG has become one of the most commonly performed procedures, but with the evolution of long-term outcomes data, the concern of weight regain and GERD after SG has been reported in several studies.26–28 A meta-analysis by Lauti et al29 suggested a multifactorial etiology for weight regain. Proposed factors included initial sleeve size, sleeve dilation, increased ghrelin levels, inadequate follow-up support, and maladaptive lifestyle behaviors. Rates of regain were found to have ranged from 5.7 percent at two-years to up to 75.6 years at six-years postsurgery. This weight regain can also be accompanied by a return of obesity-related comorbidities that might have previously resolved.30
The incidence of revisional bariatric surgery has been increasing steadily, with the most common reasons for the procedure being weight regain or insufficient weight loss.31,32 Patient selection with regard to who it should be offered to or who would benefit most from repeat surgical intervention involves a thorough evaluation by a multidisciplinary team to identify possible causes for initial failure. The key factors to consider include initial surgery and surgical anatomy, duration since index procedure, medical comorbidities, behavioral and nutritional challenges, social support, ability for reliable follow-up, and overall risk versus benefit assessment for reoperative intervention.
For high-risk patients or those with very high BMI (BMI >60kg/m2), laparoscopic SG has been proven to be a safe and effective planned first stage procedure prior to DS and/or biliopancreatic diversion.33,34 Alternatively, there might be a need for revision following a definitive SG, owing to insufficient weight loss, weight regain, or appearance of de novo gastroesophageal reflux symptoms.26,35
Of the various revision options after a SG, while the vast majority are converted to a RYGB, there have been several reports describing alternate procedures, such as DS/BPD or SADI/SADI- S.11–13,36 The experience with SADI-S has been relatively new, and long-term data on it being performed as a secondary procedure is still evolving. According to a consensus statement by the International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) taskforce in 2018, SADI-S/OADS was felt to be a safe and effective procedure, based on short-term data available.37
Since that time, additional outcomes data have emerged. As a primary procedure, most larger series (n≥100) with a follow-up of at least two years reported median percent total body weight loss (%TBWL) ranging between 22 to 48 percent,38–42 and others reported median %EWL between 58 and 86 percent.41–44 Specifically with regards to SADI-S/OADS as a secondary procedure after prior SG, available data is more sparse and mostly limited to a maximum of two-year follow-up postsurgery, with only a small amount extending beyond that.
One of the initial reports on SADI after SG with a small number of patients (n=16) by Sánchez-Pernaute et al45 in 2015 reported roughly an additional 32 %EWL following SADI, for a total of 72 %EWL at two-years postsurgery. Complete comorbidity resolution rate was reported to be 88 percent for diabetes, 60 percent for hypertension, and 40 percent for dyslipidemia. One patient suffered an isolated episode of clinical hypoalbuminemia, but otherwise it was found to be a safe operation with satisfactory results after a previously inadequate or failed SG.45
Another study by Balibrea et al46 published mid-term results with 24-month follow-up that indicated satisfactory additional %EWL of about 46 percent, for a total %EWL of 78.9±35.5 percent from index procedure. Acceptable comorbidity resolution was also noted; however, 10 percent of patients required additional revisional surgery secondary to hypoalbuminemia, stressing the importance of patient selection. Dijkhorst et al47 compared RYGB and SADI after failed SG (weight regain or insufficient weight loss) and found 8.7 percent, 12.4 percent, and 19.4 percent higher %TBWL at six, 12, and 24 months with SADI, compared to RYGB. There was a similar rate of complications and nutritional deficiencies for both groups with no intra- or postoperative mortality.47 Another short-term study with limited numbers by Ceha et al48 compared the two procedures and noted longer operating times, more defecation problems, and fewer vitamin deficiencies with SADI, but no significant differences in terms of total weight loss or comorbidity resolution.48 A multicenter retrospective review by Surve et al39 looked at complications associated with single-anastomosis/loop duodenal ileostomies, which included 123 linear-stapled and 1205 hand-sewn anastomoses. Overall anastomotic leak, ulcer, and bile reflux rate was 0.6 percent, 0.1 percent, and 0.1 percent, respectively. No patients experienced a volvulus or internal hernia. Stricture rate was 0.3 percent and 0.1 percent in the linear-stapled verses hand-sewn groups, respectively.39
With this and similar emerging data, there was an updated IFSO taskforce statement in 2020 regarding SADI-S/OADS.49 The key recommendations from this were as follows:
SADI-S/OADS offers substantial weight loss that is maintained into the mid-term.
SADI-S/OADS provides an improvement in metabolic health that is maintained into the mid-term.
Nutritional deficiencies are emerging as long-term safety concerns for the SADI-S/OADS procedure, and patients undergoing this procedure need to be aware of this risk and counseled to stay in long-term multidisciplinary care.
Surgeons performing the SADI-S/OADS, as well as other bariatric/metabolic procedures, are encouraged to participate in a national or international registry so that data may be more effectively identified.
IFSO supports the SADI-S/OADS as a recognized bariatric/metabolic procedure, but highly encourages randomized, controlled trials in the near future.
Revisional bariatric procedures are considered to be inherently more complex, and compared to primary procedures, there is a higher reported incidence of 30-day adverse events.50 A systematic review on reoperative bariatric surgery from 2014 suggested that such procedures should be performed by experienced bariatric surgeons at bariatric centers that have the necessary resources to manage challenging patients and complications in an efficient and timely manner.32
Reports regarding robotic revisions to SADI-S/OADS after prior SG are limited. We reviewed our robotic bariatric revision procedures, and of the 67 revisional procedures, 26 (38.8%) were performed after initial SG. Of these 26 revisions, four were converted to a single- or two-anastomosis DS. There were no conversions to open, and only two patients had Clavien-Dindo Grade 1 complications within 30 days.11
Qudah et al51 reported on 16 patients who underwent a robotic revisional SADI after SG with a mean operative console time of 110 minutes, no intraoperative complications, and a median hospital stay of two days. There were no perioperative reoperations, major complications, or deaths, and at median follow-up of 4.5 months, mean BMI decreased from 44kg/m2 to 38.3kg/m2, with a mean %TBWL of 12.7 percent.51
These cases are typically more challenging, due to adhesions and distorted tissue planes around the stomach and esophagus. Specifically, for SADI after SG, dense adhesions of the sleeve to the liver can be particularly difficult to manage. In addition, posterior adhesions of the sleeve to the retroperitoneum and anterior portion of the pancreas demands extremely delicate dissection. For such highly demanding procedures, we feel there is an incredible advantage with the use of the surgical robot. The stable, high-definition, three-dimensional vision is extremely useful in identifying and navigating through tough tissue planes. The EndoWrist® instruments (Intuitive Surgical, Inc.; Sunnyvale, CA, US) with expanded degrees of motion allow for increased dexterity and more efficient and versatile suturing, which typically involves a greater learning curve laparoscopically.52 Wider adoption of sutured over stapled anastomosis techniques could also help in reducing costs of surgery by limiting equipment, such as surgical staplers, and help balance other expenses related to the robot, as reported by Hagen et al.53 In a systematic review and pooled analysis on robotic versus laparoscopic RYGB by Markar et al,54 there was a reduced incidence of anastomotic strictures at six months with robotic surgery, compared to laparoscopic surgery. Even though differences in anastomotic leak rates in that pooled analysis did not reach statistical significance, a majority of studies interestingly reported lower rates associated with robotic procedures.55–59 The ability to easily utilize inbuilt fluorescence imaging adds an extra layer of safety in the evaluation of potentially tenuous vascularity at reoperative sites. The digital interface also has the ability for motion scaling, which helps negate fine tremors that might also enhance finer technical abilities. There is a reported ergonomic advantage for the surgeon in terms of reducing neck strain, back strain, and fatigue by reducing workload, compared to open or laparoscopic surgery.60
Conclusion
With increasing options and complexity in bariatric surgery, especially with revisional bariatric surgery, it is important for surgeons to be well-versed in all the possible options to provide top-quality comprehensive care. Knowledge of novel procedures is paramount, along with the ability to adopt the latest technology and improve patient outcomes.
References
- Scopinaro N, Gianetta E, Civalleri D, et al. Bilio-pancreatic bypass for obesity: II. initial experience in man. Br J Surg. 1979;66(9):618–20.
- Hess DS, Hess DW, Oakley RS. The biliopancreatic diversion with the duodenal switch: results beyond 10 years. Obes Surg. 2005;15(3):408–416.
- Regan JP, Inabnet WB, Gagner M, Pomp A. Early experience with two-stage laparoscopic Roux-en-Y gastric bypass as an alternative in the super-super obese patient. Obes Surg. 2003;13(6):861–864.
- Ren CJ, Patterson E, Gagner M. Early results of laparoscopic biliopancreatic diversion with duodenal switch: a case series of 40 consecutive patients. Obes Surg. 2000;10(6):514–523; discussion 524.
- Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2011. Obes Surg. 2013;23(4):427–436.
- American Society for Metabolic and Bariatric Surgery. Estimate of bariatric surgery numbers, 2011-2019. Mar 2021. https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers. Accessed 2 Mar 2022.
- Azagury D, Mokhtari TE, Garcia L, et al. Heterogeneity of weight loss after gastric bypass, sleeve gastrectomy, and adjustable gastric banding. Surgery. 2019;165(3):565–570.
- Lee WJ, Chong K, Ser KH, et al. Gastric bypass vs sleeve gastrectomy for Type 2 diabetes mellitus: a randomized controlled trial. Arch Surg. 2011;146(2):143–148.
- Peterli R, Wölnerhanssen BK, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA. 2018;319(3):255–265.
- Vilallonga R, Fort JM, Caubet E, et al. Robotically assisted single anastomosis duodenoileal bypass after previous sleeve gastrectomy implementing high valuable technology for complex procedures. J Obes. 2015;2015:586419.
- Cheng YL, Elli EF. Role of robotic surgery in complex revisional bariatric procedures. Obes Surg. 2021;31(6):2583–2589.
- Carmeli I, Golomb I, Sadot E, et al. Laparoscopic conversion of sleeve gastrectomy to a biliopancreatic diversion with duodenal switch or a Roux-en-Y gastric bypass due to weight loss failure: our algorithm. Surg Obes Relat Dis. 2015;11(1):79–85.
- Lazzati A, Bechet S, Jouma S, et al. Revision surgery after sleeve gastrectomy: a nationwide study with 10 years of follow-up. Surg Obes Relat Dis. 2020;16(10):1497–1504.
- Sánchez-Pernaute A, Rubio Herrera MA, Pérez-Aguirre E, et al. Proximal duodenal-ileal end-to-side bypass with sleeve gastrectomy: proposed technique. Obes Surg. 2007;17(12):1614–1618.
- Sánchez-Pernaute A, Herrera MA, Pérez-Aguirre ME, et al. Single anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S). One to three-year follow-up. Obes Surg. 201020(12):1720–1726.
- Vilallonga R, Nedelcu A, Cirera de Tudela A, et al. Single anastomosis duodeno-ileal bypass as a revisional procedure following sleeve gastrectomy: review of the literature. J Laparoendosc Adv Surg Tech A. 2021 Sep 27. Online ahead of print.
- Gray KD, Moore MD, Elmously A, et al. Perioperative outcomes of laparoscopic and robotic revisional bariatric surgery in a complex patient population. Obes Surg. 2018;28(7):1852–1859.
- Bindal V, Gonzalez-Heredia R, Elli EF. Outcomes of robot-assisted Roux-en-Y gastric bypass as a reoperative bariatric procedure. Obes Surg. 2015;25(10):1810–1815.
- Prachand VN, Ward M, Alverdy JC. Duodenal switch provides superior resolution of metabolic comorbidities independent of weight loss in the super-obese (BMI > or = 50 kg/m2) compared with gastric bypass. J Gastrointest Surg. 2010;14(2):211–220.
- Skogar ML, Sundbom M. Duodenal switch is superior to gastric bypass in patients with super obesity when evaluated with the Bariatric Analysis and Reporting Outcome System (BAROS). Obes Surg. 2017;27(9):2308–2316.
- Hedberg J, Sundström J, Sundbom M. Duodenal switch versus Roux-en-Y gastric bypass for morbid obesity: systematic review and meta-analysis of weight results, diabetes resolution and early complications in single-centre comparisons. Obes Rev. 2014;15(7):555–563.
- Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357(8):753–761.
- Sjöström L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351(26):2683–2693.
- Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–1737.
- Adams TD, Davidson LE, Litwin SE, et al. Weight and metabolic outcomes 12 years after gastric bypass. N Engl J Med. 2017;377(12):1143–1155.
- Himpens J, Dobbeleir J, Peeters G. Long-term results of laparoscopic sleeve gastrectomy for obesity. Ann Surg. 2010;252(2):319–324.
- Lemanu DP, Singh PP, Rahman H, et al. Five-year results after laparoscopic sleeve gastrectomy: a prospective study. Surg Obes Relat Dis. 2015;11(3):518–524.
- Saif T, Strain GW, Dakin G, et al. Evaluation of nutrient status after laparoscopic sleeve gastrectomy 1, 3, and 5 years after surgery. Surg Obes Relat Dis. 2012;8(5):542–547.
- Lauti M, Kularatna M, Hill AG, MacCormick AD. Weight regain following sleeve gastrectomy-a systematic review. Obes Surg. 2016;26(6):1326–1334.
- King WC, Hinerman AS, Belle SH, et al. Comparison of the performance of common measures of weight regain after bariatric surgery for association with clinical outcomes. JAMA. 2018;320(15):1560–1569.
- Gagner M, Gumbs AA. Gastric banding: conversion to sleeve, bypass, or DS. Surg Endosc. 2007;21(11):1931–1935.
- Brethauer SA, Kothari S, Sudan R. Systematic review on reoperative bariatric surgery: American Society for Metabolic and Bariatric Surgery Revision Task Force. Surg Obes Relat Dis. 2014;10(5):952–972.
- Brethauer SA, Hammel JP, Schauer PR. Systematic review of sleeve gastrectomy as staging and primary bariatric procedure. Surg Obes Relat Dis. 2009;5(4):469–475.
- Cottam D, Qureshi FG, Mattar SG, et al. Laparoscopic sleeve gastrectomy as an initial weight-loss procedure for high-risk patients with morbid obesity. Surg Endosc. 2006;20(6):859–863.
- Shimizu H, Annaberdyev S, Motamarry I, et al. Revisional bariatric surgery for unsuccessful weight loss and complications. Obes Surg. 2013;23(11):1766–1773.
- Andalib A, Alamri H, Almuhanna Y, et al. Short-term outcomes of revisional surgery after sleeve gastrectomy: a comparative analysis of re-sleeve, Roux en-Y gastric bypass, duodenal switch (Roux en-Y and single-anastomosis). Surg Endosc. 2021;35(8):4644–4652.
- Brown WA, Ooi G, Higa K, et al. Single anastomosis duodenal-ileal bypass with sleeve gastrectomy/one anastomosis duodenal switch (SADI-S/OADS) IFSO position statement. Obes Surg. 2018;28(5):1207–1216.
- Mitzman B, Cottam D, Goriparthi R, et al. Stomach intestinal pylorus sparing (SIPS) surgery for morbid obesity: retrospective analyses of our preliminary experience. Obes Surg. 2016;26(9):2098–2104.
- Surve A, Cottam D, Sánchez-Pernaute A, et al. The incidence of complications associated with loop duodeno-ileostomy after single-anastomosis duodenal switch procedures among 1328 patients: a multicenter experience. Surg Obes Relat Dis. 2018;14(5):594–601.
- Moon RC, Kirkpatrick V, Gaskins L, et al. Safety and effectiveness of single- versus double-anastomosis duodenal switch at a single institution. Surg Obes Relat Dis. 2019;15(2):245–252.
- Finno P, Osorio J, García-Ruiz-de-Gordejuela A, et al. Single versus double-anastomosis duodenal switch: single-site comparative cohort study in 440 consecutive patients. Obes Surg. 2020;30(9):3309–3316.
- Ser KH, Lee WJ, Chen JC, et al. Laparoscopic single-anastomosis duodenal-jejunal bypass with sleeve gastrectomy (SADJB-SG): surgical risk and long-term results. Surg Obes Relat Dis. 2019;15(2):236–243.
- Cottam A, Cottam D, Zaveri H, et al. An analysis of mid-term complications, weight loss, and Type 2 diabetes resolution of stomach intestinal pylorus-sparing surgery (SIPS) versus Roux-en-Y gastric bypass (RYGB) with three-year follow-up. Obes Surg. 2018;28(9):2894–2902.
- Zaveri H, Surve A, Cottam D, et al. Mid-term 4-year outcomes with single anastomosis duodenal-ileal bypass with sleeve gastrectomy surgery at a single US center. Obes Surg. 2018;28(10):3062–3072.
- Sánchez-Pernaute A, Rubio MÁ, Conde M, et al. Single-anastomosis duodenoileal bypass as a second step after sleeve gastrectomy. Surg Obes Relat Dis. 2015;11(2):351–355.
- Balibrea JM, Vilallonga R, Hidalgo M, et al. Mid-term results and responsiveness predictors after two-step single-anastomosis duodeno-ileal bypass with sleeve gastrectomy. Obes Surg. 2017;27(5):1302–1308.
- Dijkhorst PJ, Boerboom AB, Janssen IMC, et al. Failed sleeve gastrectomy: single anastomosis duodenoileal bypass or Roux-en-Y gastric bypass? A multicenter cohort study. Obes Surg. 2018;28(12):3834–3842.
- Ceha CMM, van Wezenbeek MR, Versteegden DPA, et al. Matched short-term results of SADI versus GBP after sleeve gastrectomy. Obes Surg. 2018;28(12):3809–3814.
- Brown WA, de Leon Ballesteros GP, Ooi G, et al. Single anastomosis duodenal-ileal bypass with sleeve gastrectomy/one anastomosis duodenal switch (SADI-S/OADS) IFSO position statement–update 2020. Obes Surg. 2021;31(1):3–25.
- Inabnet WB III, Belle SH, Bessler M, et al. Comparison of 30-day outcomes after non-LapBand primary and revisional bariatric surgical procedures from the Longitudinal Assessment of Bariatric Surgery study. Surg Obes Relat Dis. 2010;6(1):22–30.
- Qudah Y, Alhareb A, Barajas-Gamboa JS, et al. Robotic revisional single anastomosis duodenoileal bypass after sleeve gastrectomy. J Laparoendosc Adv Surg Tech A. 2021 Sep 7. Online ahead of print.
- Leijte E, de Blaauw I, Van Workum F, et al. Robot assisted versus laparoscopic suturing learning curve in a simulated setting. Surg Endosc. 2020;34(8):3679–3689.
- Hagen ME, Pugin F, Chassot G, et al. Reducing cost of surgery by avoiding complications: the model of robotic Roux-en-Y gastric bypass. Obes Surg. 2012;22(1):52–61.
- Markar SR, Karthikesalingam AP, Venkat-Ramen V, et al. Robotic vs. laparoscopic Roux-en-Y gastric bypass in morbidly obese patients: systematic review and pooled analysis. Int J Med Robot. 2011;7(4):393–400.
- Artuso D, Wayne M, Grossi R. Use of robotics during laparoscopic gastric bypass for morbid obesity. JSLS. 2005;9(3):266–268.
- Hubens G, Balliu L, Ruppert M, et al. Roux-en-Y gastric bypass procedure performed with the da Vinci robot system: is it worth it? Surg Endosc. 2008;22(7):1690–1696.
- Mohr CJ, Nadzam GS, Curet MJ. Totally robotic Roux-en-Y gastric bypass. Arch Surg. 2005;140(8):779–786.
- Scozzari G, Rebecchi F, Millo P, et al. Robot-asststed gastrojejunal anastomosis does not improve the results of the laparoscopic Roux-en-Y gastric bypass. Surg Endosc. 2011;25(2):597–603.
- Snyder BE, Wilson T, Leong BY, et al. Robotic-assisted Roux-en-Y gastric bypass: minimizing morbidity and mortality. Obes Surg. 2010;20(3):265–270.
- Wee IJY, Kuo LJ, Ngu JC. A systematic review of the true benefit of robotic surgery: ergonomics. Int J Med Robot. 2020;16(4):e2113.
Category: Past Articles, Review



