Effects of Lisdexamfetamine Dimesylate on Functional Impairment Measured on the Sheehan Disability Scale in Adults With Moderate-to-severe Binge Eating Disorder: Results from Two Randomized, Placebo-controlled Trials
 by David V. Sheehan, MD, MBA; Maria Gasior, MD, PhD; Susan L. McElroy, MD; Jana Radewonuk, MSc; Barry K. Herman, MD, MMM; and James Hudson, MD, ScD
by David V. Sheehan, MD, MBA; Maria Gasior, MD, PhD; Susan L. McElroy, MD; Jana Radewonuk, MSc; Barry K. Herman, MD, MMM; and James Hudson, MD, ScD
Dr. Sheehan is with the University of South Florida College of Medicine in Tampa, Florida. Drs. Herman and Gasior and Ms. Radewonuk are formerly affiliated with Shire Pharmaceuticals in Lexington, Massachusetts. Dr. McElroy is with the Lindner Center of HOPE and the University of Cincinnati College of Medicine in Mason, Ohio. Dr. Hudson is with McLean Hospital/Harvard Medical School in Belmont, Massachusetts.
FUNDING: This clinical research was supported by the sponsor, Shire Development LLC (Lexington, Massachusetts). The sponsor was involved in the design and conduct of the study, in the collection and analysis of the data, and in the development of the manuscript. The final content and decision to submit this manuscript to Innovations in Clinical Neuroscience was made by the authors. Shire Development LLC provided funding to Complete Healthcare Communications, LLC (CHC) for support in writing and editing this manuscript.
DISCLOSURES: Dr. Sheehan is a consultant to Shire Pharmaceuticals and the author of the Sheehan Disability Scale, which measures functional impairment and is the focus of this manuscript; he is an advisory member of the editorial board for Innovations and Clinical Neuroscience. Dr. Gasior is a former employee of Shire and a current employee of BTG International; she holds stock and/or stock options in Shire and BTG International. Ms. Radewonuk is a former employee of Shire and a current employee of The Griesser Group; she holds stock and/or stock options in Shire. Dr. McElroy is a consultant to and has received grant support from Shire. In addition, she is also a consultant to or member of the scientific advisory boards of Alkermes, Bracket, Corcept, F. Hoffmann-LaRoche Ltd, MedAvante, Myriad, Naurex, Novo Nordisk, Sunovion, and Teva. She has also received grant support from the Agency for Healthcare Research & Quality (AHRQ), Alkermes, AstraZeneca, Cephalon (now Teva), Forest, Lilly, Marriott Foundation, the National Institute of Mental Health, Orexigen, Pfizer, Takeda, and Transcept. She is also an inventor on United States Patent No. 6,323,236 B2, Use of Sulfamate Derivatives for Treating Impulse Control Disorders and, along with the patent’s assignee (University of Cincinnati; Cincinnati, Ohio), has received payments from Johnson & Johnson, which has exclusive rights under the patent. Dr. Herman is a former employee of Shire and holds stock and/or stock options in Shire; he is currently an employee of Tris Pharma (Monmouth Junction, New Jersey). Dr. Hudson has received consulting fees and grant support from Shire. In addition, he has received consulting fees from diaMentis, Genentech, and Sunovion as well as grant support from Genentech and Sunovion.
Reprinted with permission from Innov Clin Neurosci. 2018;15(5–6):22–29.
© Copyright Matrix Medical Communications, 2018.
Abstract
Objective: In two Phase III, randomized, placebo-controlled trials (NCT01718483 and NCT01718509 at ClinicalTrials.gov), lisdexamfetamine dimesylate (LDX) reduced binge eating days/week in adults with moderate-to-severe binge eating disorder (BED). We describe the effects of LDX (50mg and 70mg) on the Sheehan Disability Scale (SDS; exploratory endpoint) from both studies. Design: The SDS was assessed at baseline, Week 6, and Week 12/early termination. Analyses included mixed-effects models for repeated measures for the examination of SDS total and domain score changes and a generalized estimating equation model to assess dichotomized remission status (remission [total score ≤6] versus nonremission [total score >6]). Results: Least squares (95% confidence interval [CI]) mean treatment differences for SDS total score change from baseline at Week 12 were -2.80 (-3.98, -1.61) in Study 1 and -3.70 (-4.81, -2.58) in Study 2 (both p<0.001). Least squares (95% CI) mean treatment differences across SDS domains favored LDX over placebo in both studies for the change from baseline at Week 12 (work/school: -0.8 [-1.2, -0.4] and -1.1 [-1.5, -0.7], both p<0.001; social life/leisure activities: -1.0 [-1.4, -0.5] and -1.4 [-1.8, -1.0], both p<0.001; and family life/home responsibilities: -1.0 [-1.4, -0.5] and -1.3 [-1.7, -0.9], both p<0.001). Odds ratios (95% CI) for SDS remission versus nonremission favored LDX over placebo at Week 12 (Study 1: 2.39 [1.44, 3.96]; p<0.001 and Study 2: 5.12 [2.80, 9.33]; p<0.001). Conclusion: These findings indicate that LDX treatment is associated with improvement on the SDS in adults with moderate-to-severe BED.
Keywords: binge eating disorder, disability, functionality, lisdexamfetamine dimesylate, Sheehan Disability Scale
Introduction
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) recognizes binge eating disorder (BED) as a distinct eating disorder.1 Left untreated, individuals with BED often experience reduced quality of life2 and impaired functionality3 in multiple domains, including work/school, social life, and family responsibilities, compared to individuals without BED. For instance, on the Sheehan Disability Scale (SDS), a validated discretized analog (Discan) scale that assesses functional impairment in the work/school, social life/leisure activities, and family life/home responsibilities domains,4,5 role impairment (i.e., an individual’s inability to fulfill expected roles in different aspects of his or her life) was reported in individuals meeting DSM, Fourth Edition, Text Revision (DSM-IV-TR), criteria for BED (any role impairment: 46.7% and severe role impairment: 13.2%).3
The short-term efficacy, safety, and tolerability of lisdexamfetamine dimesylate (LDX) in adults with moderate-to-severe BED have been examined in two placebo (PBO)-controlled, double-blind, Phase III studies. In these pivotal studies, dose-optimized LDX (50mg and 70mg) produced statistically greater reductions in binge-eating days per week (primary efficacy endpoint) than did PBO in adults with protocol-defined moderate-to-severe BED.6 LDX was also associated with significantly greater global improvement on the Clinical Global Impressions–Improvement scale, four-week cessation of binge eating at endpoint, reductions in BED-related obsessive and compulsive psychopathology on the Yale-Brown Obsessive Compulsive Scale Modified for Binge Eating, percentage body weight change, and reductions in triglycerides (key secondary endpoints) as compared to PBO in both studies.6 Similarly, LDX (50mg and 70mg) demonstrated efficacy versus PBO in a Phase II, double-blind, fixed-dose study in adults with moderate-to-severe BED, as demonstrated by greater decreases in binge-eating days per week.7
In these short-term studies,6,7 the safety and tolerability profile of LDX was similar to its established safety profile for the treatment of attention deficit hyperactivity disorder (ADHD).8 Treatment-emergent adverse events reported by 10 percent or more of participants with BED treated with LDX in any study were dry mouth, decreased appetite, insomnia, and headache.7 Across all three short-term BED studies,6,7 observed small increases in blood pressure and heart rate were also consistent with the established safety profile of LDX for ADHD.8
The objective of this report is to describe the effects of LDX on functional impairment as measured by changes on the SDS, which was assessed as an exploratory endpoint in the pivotal Phase III trials.6 The SDS has been validated9–12 and used to assess functional disability13–18 in multiple patient populations. Although the SDS has not been clinically validated in individuals with BED, it has demonstrated internal consistency, test–retest reliability, and construct validity across many different populations.5,9–12
Methods
The overall methodology of the two Phase III BED studies discussed in this report has previously been described in detail.6 This information is briefly summarized here.
Participants. Eligible participants were 18 to 55 years of age, met the DSM-IV-TR BED criteria,19 exhibited protocol-defined moderate-to-severe BED (defined as 3 or more binge eating days each week for the 14 days before the baseline assessment), and had a Clinical Global Impressions–Severity score of 4 or greater at screening and baseline.
Key exclusion criteria included a current diagnosis of anorexia nervosa or bulimia nervosa; a comorbid psychiatric disorder controlled with study-prohibited medications or uncontrolled and associated with significant symptoms; any condition or symptom that might confound study assessments; the use of psychotherapy or weight loss support for BED within three months of screening; a previous suicide attempt, current demonstration of active suicidal ideation, or potential suicide risk as determined by the investigator; a history of cardiovascular conditions, moderate or severe hypertension, average sitting systolic blood pressure greater than 139mmHg, or an average diastolic blood pressure greater than 89mmHg; a lifetime history of stimulant abuse or recent history of substance abuse or dependence; or a known or suspected intolerance of or hypersensitivity to LDX or related compounds.
Study design and treatment. The two randomized, PBO-controlled, multicenter studies (NCT01718483 conducted between November 26, 2012, and September 25, 2013, at 50 clinical research sites and NCT01718509 conducted between November 26, 2012, and September 20, 2013, at 43 clinical research sites) described in this report were conducted using the same design and methods.6 Each study included a screening phase; a 12-week double-blind phase (dose optimization, 4 weeks; dose maintenance, 8 weeks); and a one-week follow-up phase. Study protocols for both studies were approved by ethics committees, and both studies were conducted in accordance with the International Conference on Harmonisation Good Clinical Practice guidelines and the principles of the Declaration of Helsinki. Participants were required to provide written, informed consent before entering the studies.
After screening, participants were randomized 1:1 to 12 weeks of dose-optimized LDX or matching PBO, with both the participant and investigator blinded to treatment. To maintain blinding, both treatments were identical in appearance. Treatment started at 30mg LDX during Week 1; the dosage was titrated to 50mg LDX during Week 2. During Weeks 3 and 4, dosage increases to 70mg LDX were made based on tolerability and clinical need. A single titration down to 50mg LDX was allowed during Week 3 based on participant tolerability. During Weeks 4 to 12, the optimized LDX dosage (50mg or 70mg) was maintained. If a dosage reduction occurred during the optimization phase, no further changes were allowed during maintenance; participants requiring such a reduction were discontinued. A follow-up visit occurred one week after the Week 12/early termination (ET) visit to assess safety.
Sheehan Disability Scale. In both studies, functionality was assessed as an exploratory endpoint using the SDS. Assessments of the SDS occurred at baseline, Week 6, and Week 12/ET. On the SDS, participants rated the effect of their BED on work/school, social life/leisure activities, and family life/home responsibilities using an 11-point Discan scale (0=no impairment, 1–3=mild, 4–6=moderate, 7–9=marked, and 10=extreme); total scores ranged from 0 to 30.4 The numbers of days lost from work/school and days of underproductivity while at work/school were also assessed using the SDS instrument.20
Data presentation and statistical analyses. Sample size in each study was determined based on the primary efficacy endpoint of change from baseline in binge eating days/week at Weeks 11 to 12.6 None of the analyses described in the current report were included as part of the prespecified hierarchical testing strategy for controlling multiplicity in either study. As such, all reported p-values are nominal (unadjusted for multiplicity and descriptive).
Least squares (LS) mean treatment differences (LDX-PBO) in the change from baseline SDS total score (prespecified analysis), SDS domain scores (prespecified analyses), and for the numbers of days lost and days underproductive (individually and combined [post-hoc analyses]) while at work/school in the last week were separately assessed in the full analysis set (FAS; all randomized participants taking 1 or more study drug doses and having 1 or more post-baseline assessments of the number of binge eating days per week) of each study. Data were analyzed using a mixed-effects model for repeated measures with treatment, visit, and the treatment x visit interaction as factors, baseline score as a covariate, and its interaction with visit also in the model using an unstructured covariance matrix. Effect size was calculated based on the estimated standard deviation from the unstructured covariance matrix.
Based on findings of several previous studies,4,14,15 an SDS remission analysis was also conducted. In this post-hoc analysis, SDS total scores were dichotomized as indicating remission (SDS total score ≤6; minimal or no functional disability) or nonremission (SDS total score >6; some level of functional disability) at baseline and Week 12. Dichotomized remission status was analyzed using a generalized estimating equation model over all visits with remission status as the outcome variable, and treatment, visit, and the treatment x visit interaction as factors using an unstructured covariance matrix; odds ratios for remission versus nonremission were calculated as LDX versus PBO.
Results
Participant disposition and demographics. Participant disposition in each study is described in detail elsewhere.6 In brief, 383 participants were randomized in Study 1 (PBO, n=191 and LDX, n=192) and 390 participants were randomized in Study 2 (PBO, n=195 and LDX, n=195). The FAS included 374 participants in Study 1 (PBO, n=184 and LDX, n=190) and 350 participants in Study 2 (PBO, n=176 and LDX, n=174). Sixty-eight participants did not complete Study 1 (PBO, n=34 and LDX, n=34) and 96 participants did not complete Study 2 (PBO, n=48 and LDX, n=48). The most frequent reasons for discontinuation in Study 1 were participant withdrawal in the PBO treatment arm (n=14) and adverse events and participant withdrawal in the LDX treatment arm (both n=12); in Study 2, the most common reasons were lost to follow-up in the PBO treatment arm (n=18) and in the LDX treatment arm (n=15). Key participant demographics and clinical characteristics in the FAS are summarized in Table 1. 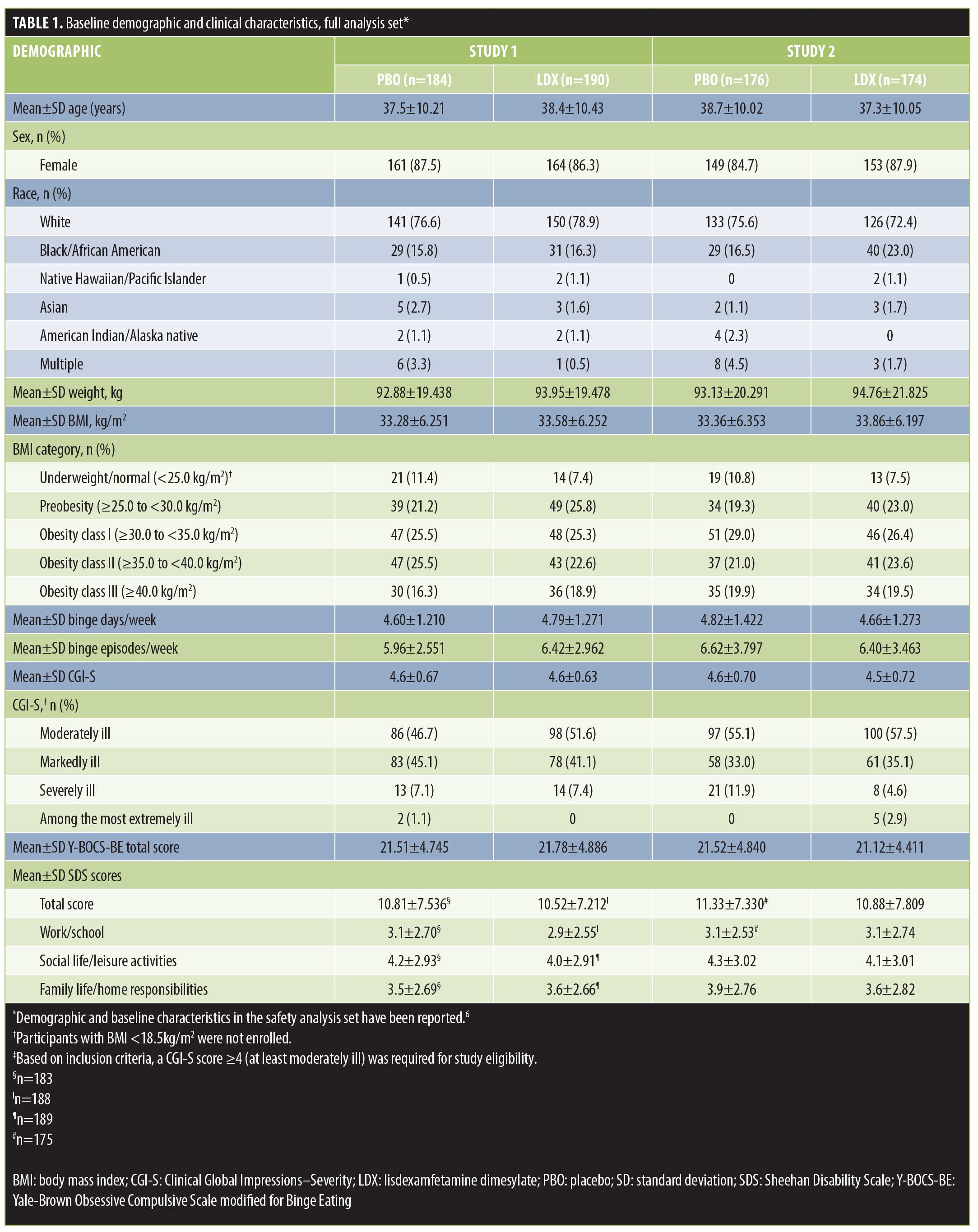
Sheehan Disability Scale total score. Baseline SDS total scores are presented in Table 1. Mean ± standard deviation (SD) SDS total scores decreased from baseline for both treatment groups during double-blind treatment (Figure 1). In both studies, the LS mean (95% confidence interval [CI]) treatment difference for the SDS total score change from baseline favored LDX over PBO at Week 6 (Study 1: -3.06 [-4.18, -1.95]; effect size=0.57 and Study 2: -3.48 [-4.57, -2.38]; effect size=0.69; both p<0.001) and Week 12 (Study 1: -2.80 [-3.98, -1.61]; effect size=0.51 and Study 2: -3.70 [-4.81, -2.58]; effect size=0.74; both p<0.001).
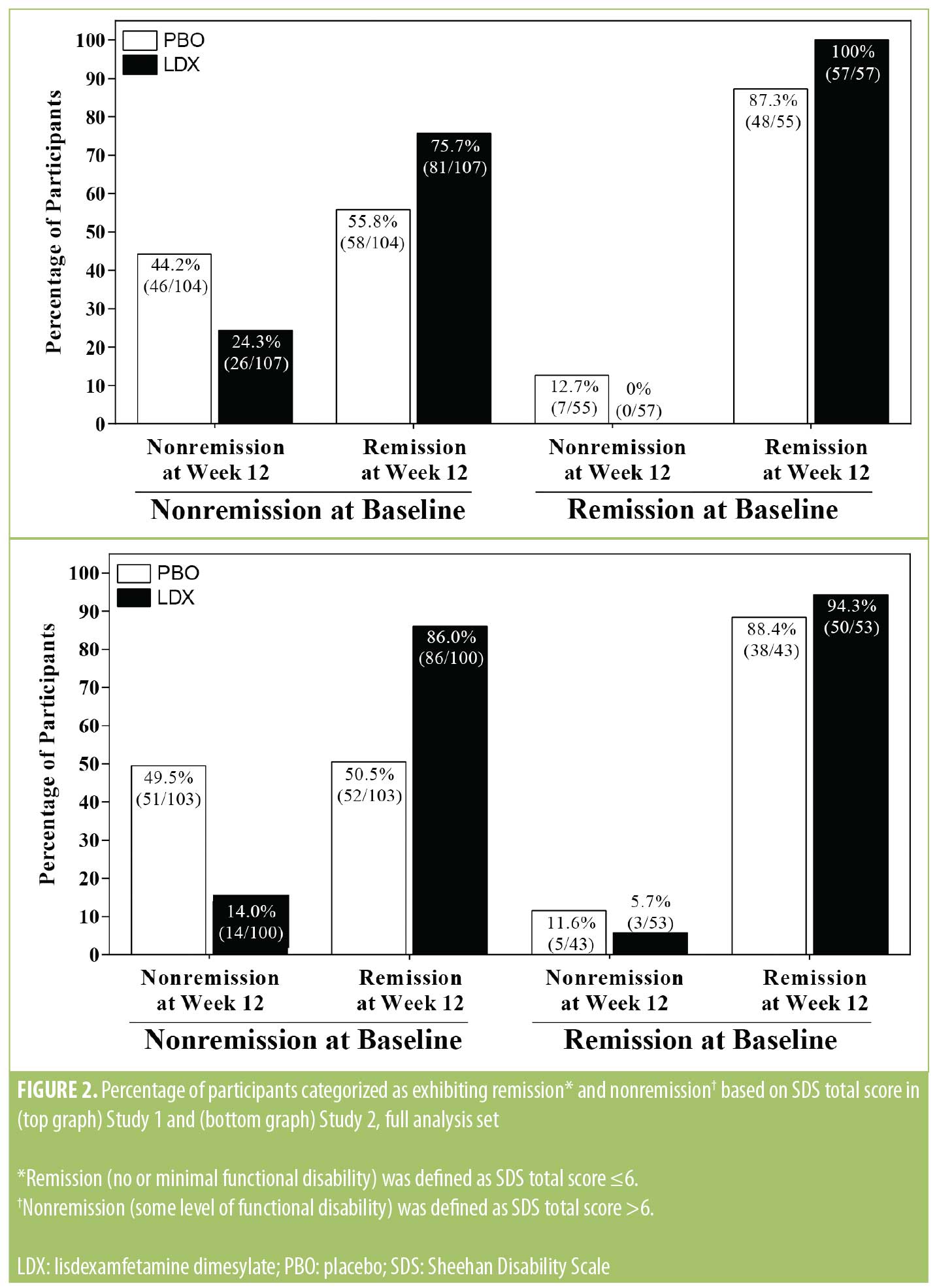 Sheehan Disability Scale remission analysis. At baseline, lower percentages of participants were categorized as meeting the criterion for SDS remission versus nonremission (based on SDS total score) in Study 1 in both the PBO (remission: 35.0% [64/183] and nonremission: 65.0% [119/183]) and LDX (remission: 34.6% [65/188] and nonremission: 65.4% [123/188]) treatment arms, and, in Study 2, in both the PBO (remission: 29.1% [51/175] and nonremission: 70.9% [124/175]) and LDX (remission: 35.6% [62/174] and 64.4% [112/174]) treatment arms. Percentages of participants meeting the criterion for SDS remission and nonremission at Week 12 as a function of baseline nonremission or remission status are depicted in Figure 2. Among participants meeting the criterion for nonremission at baseline, higher percentages of participants met the criterion for SDS remission than nonremission at Week 12 in both studies in each treatment group. Among participants meeting the criterion for remission at baseline, most participants in both studies in each treatment group continued to meet the remission criterion at Week 12. The odds ratio (95% CI) for remission versus nonremission at Week 12 favored LDX over PBO in both studies (Study 1: 2.39 [1.44, 3.96]; p<0.001 and Study 2: 5.12 [2.80, 9.33]; p<0.001).
Sheehan Disability Scale remission analysis. At baseline, lower percentages of participants were categorized as meeting the criterion for SDS remission versus nonremission (based on SDS total score) in Study 1 in both the PBO (remission: 35.0% [64/183] and nonremission: 65.0% [119/183]) and LDX (remission: 34.6% [65/188] and nonremission: 65.4% [123/188]) treatment arms, and, in Study 2, in both the PBO (remission: 29.1% [51/175] and nonremission: 70.9% [124/175]) and LDX (remission: 35.6% [62/174] and 64.4% [112/174]) treatment arms. Percentages of participants meeting the criterion for SDS remission and nonremission at Week 12 as a function of baseline nonremission or remission status are depicted in Figure 2. Among participants meeting the criterion for nonremission at baseline, higher percentages of participants met the criterion for SDS remission than nonremission at Week 12 in both studies in each treatment group. Among participants meeting the criterion for remission at baseline, most participants in both studies in each treatment group continued to meet the remission criterion at Week 12. The odds ratio (95% CI) for remission versus nonremission at Week 12 favored LDX over PBO in both studies (Study 1: 2.39 [1.44, 3.96]; p<0.001 and Study 2: 5.12 [2.80, 9.33]; p<0.001).
Sheehan Disability Scale domain scores. Baseline SDS domain scores are presented in Table 1. Mean ± SD SDS domain scores decreased from baseline in both treatment groups during double-blind treatment in both studies (Figure 3). During double-blind treatment, LS mean (95% CI) treatment differences for the change from baseline favored LDX over PBO on all SDS domains in both studies (Study 1 and Study 2, respectively) at Week 6 (work/school: -0.9 [-1.3, -0.6] and -1.1 [-1.5, -0.7], effect sizes=0.51 and 0.64, both p<0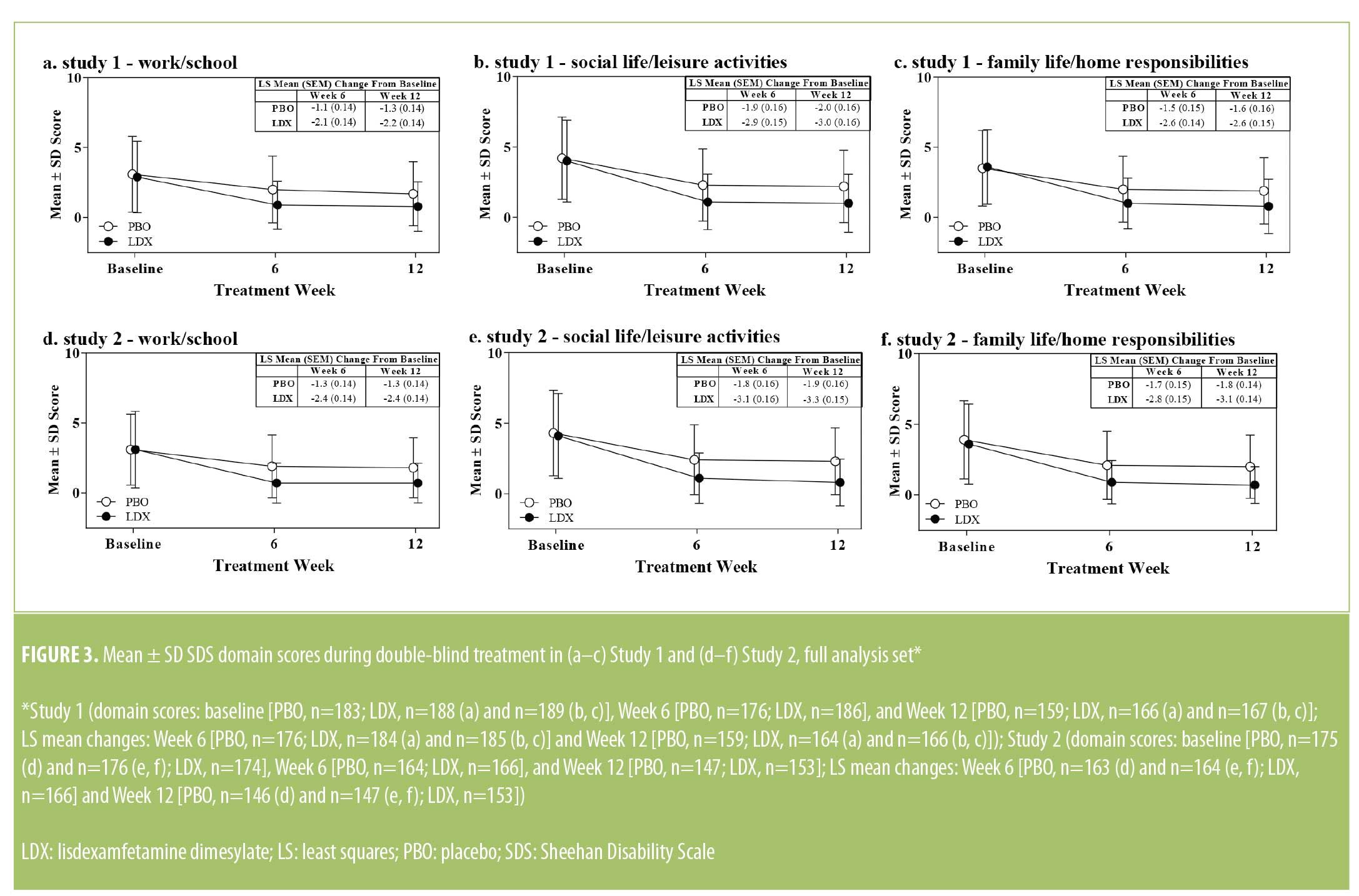 .001; social life/leisure activities: -1.1 (-1.5, -0.7) and -1.3 (-1.7, -0.8), effect sizes=0.53 and 0.64, both p<0.001; family life/home responsibilities: -1.1 [-1.5, -0.7] and -1.2 [-1.6, -0.8], effect sizes=0.55 and 0.62, both p<0.001] and Week 12 (work/school: -0.8 [-1.2, -0.4] and -1.1 [-1.5, -0.7], effect sizes=0.46 and 0.64, both p<0.001; social life/leisure activities: -1.0 [-1.4, -0.5] and -1.4 [-1.8, -1.0], effect sizes=0.46 and 0.72, both p<0.001; family life/home responsibilities: -1.0 [-1.4, -0.5] and -1.3 [-1.7, -0.9], effect sizes=0.47 and 0.72, both p<0.001).
.001; social life/leisure activities: -1.1 (-1.5, -0.7) and -1.3 (-1.7, -0.8), effect sizes=0.53 and 0.64, both p<0.001; family life/home responsibilities: -1.1 [-1.5, -0.7] and -1.2 [-1.6, -0.8], effect sizes=0.55 and 0.62, both p<0.001] and Week 12 (work/school: -0.8 [-1.2, -0.4] and -1.1 [-1.5, -0.7], effect sizes=0.46 and 0.64, both p<0.001; social life/leisure activities: -1.0 [-1.4, -0.5] and -1.4 [-1.8, -1.0], effect sizes=0.46 and 0.72, both p<0.001; family life/home responsibilities: -1.0 [-1.4, -0.5] and -1.3 [-1.7, -0.9], effect sizes=0.47 and 0.72, both p<0.001).
Days lost and of underproductivity at work/school. The numbers of days lost and of underproductivity at work/school in the last week are presented, both individually and combined, in Table 2. At baseline, the numbers of days lost, days of underproductivity, and the combined value for days lost and of underproductivity in the last week were comparable between treatment arms in each study. Mean ± SD reductions at Week 12 were observed for all measures in both treatment arms in each study. At Week 12, LS mean treatment differences favored LDX over PBO for days of underproductivity and the combination of days lost and days of underproductivity in both studies, as well as for days lost in Study 2 (Table 2; all p<0.05).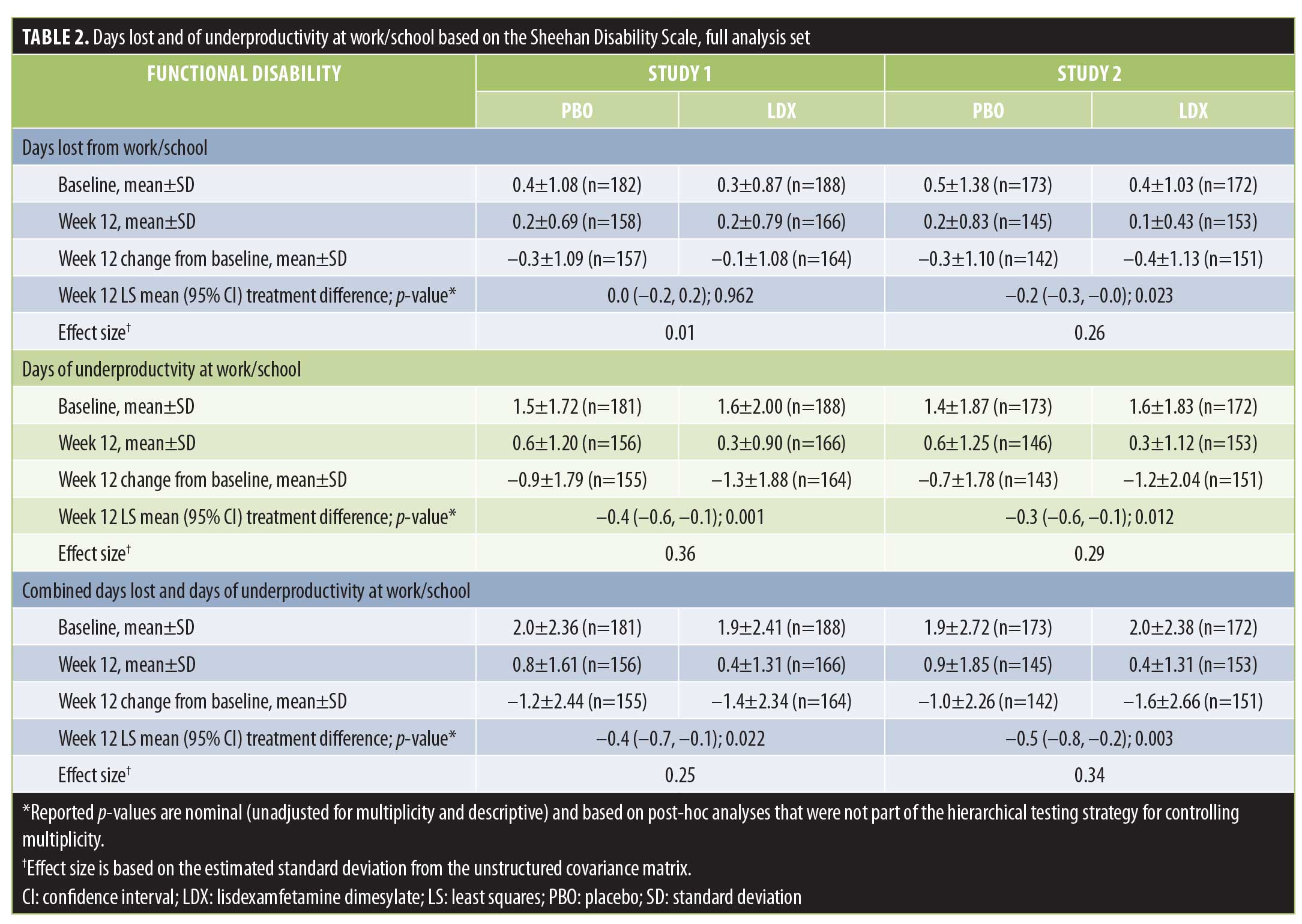
Discussion
In two large-scale PBO-controlled trials, reductions in functional disability as measured by SDS total score at Week 12 favored LDX over PBO in adults with moderate-to-severe BED. In support of this finding, greater improvement in functioning was also observed for LDX over PBO for SDS domain scores at Week 12, with effect sizes being moderate, and for days of underproductivity at work/school in the last seven days, with effect sizes being small. Treatment with LDX was also associated with numerically greater rates of SDS remission at Week 12. These results suggest that LDX treatment was associated with clinically relevant improvement in functioning in adults with moderate-to-severe BED.
In these two studies, mean baseline SDS scores in study participants were indicative of mild-to-moderate functional disability across all domains.4 Based on mean values at baseline, the current analyses also suggest that BED was more likely to be associated with underproductivity at work/school than with days lost from work/school. Further supporting the notion that a substantial portion of study participants exhibited some level of functional disability at baseline, approximately two-thirds of study participants were categorized as meeting criteria for nonremission based on SDS total score. Because individuals with psychiatric comorbidities were excluded, the observed disability at baseline can be attributed to BED.
These findings are consistent with other published data relating to functional impairment in individuals with BED. In a study of women conducted at primary care and gynecology clinics throughout the United States, BED was associated with statistically significantly worse function on all six indices (i.e., physical functioning, social functioning, role functioning, mental health, bodily pain, and general health perception) of the Medical Outcomes Study Short-Form General Health Survey in comparison with women without psychiatric disorders.21 Similarly, in a publication based on World Health Organization (WHO) World Mental Health Surveys, impaired functionality in individuals with BED was evidenced by the fact that early-onset BED was associated with reduced odds of being employed among men (but not women).22 In another publication based on the WHO World Mental Health Surveys—which incorporated findings from the National Comorbidity Survey Replication conducted in the United States23 and the European Study of the Epidemiology of Mental Disorders conducted in six European countries24—role impairment (i.e., an individual’s inability to fulfill expected roles in different aspects of life) as measured across all SDS domains was reported in individuals meeting DSM-IV-TR criteria for BED (any role impairment: 46.7%; severe role impairment: 13.2%).3
The level of functional impairment exhibited on the SDS by individuals with BED at baseline in these studies is also consistent with previously published clinical studies.25,26 In a randomized, PBO-controlled study of topiramate in individuals with BED,25 baseline SDS total score and domain scores were comparable to those reported in these two studies. However, SDS domain scores in an open-label, prospective study of memantine26 tended to be lower than scores in the topiramate and LDX studies, despite having generally similar levels of binge-eating days per week and binge-eating episodes per week. As was observed in this report, the studies of topiramate25 and memantine26 also indicated that impairment tended to be higher in the social life/leisure activities and family life/home responsibilities domains than in the work/school domain.
The reductions in functional impairment associated with LDX treatment that were observed in the current studies are also generally consistent with the effects of topiramate and memantine on functional impairment.25,26 In the topiramate study, statistically greater improvements in SDS total score, the social life domain score, and the family life domain score were observed with topiramate than with PBO in adults with BED.25 In an open-label, prospective study, memantine produced statistically significant reductions from baseline in all SDS domain scores in adults with BED.26
At the time of this study, there were no published reports of the effect of pharmacotherapy on days lost or underproductive at school/work or on SDS remission rates in adults with BED. The findings from these studies for these endpoints suggest that LDX was associated with improved functionality in study participants, with the effects of LDX more consistently improving underproductivity than days lost. Furthermore, the odds ratios for remission versus nonremission at Week 12 in both studies favored LDX over PBO. Taken together, these findings suggest that improvement on the SDS favored LDX over PBO.
Limitations. When interpreting these data, several limitations should be considered. First, the SDS was an exploratory endpoint in these studies and was not part of the hierarchical testing strategy for controlling for multiplicity. Therefore, all reported p-values for both prespecified and post-hoc analyses based on the SDS are reported for descriptive purposes only. Second, although the SDS has been validated9–12 and used to assess functional disability13–18 in multiple patient populations, it has not been clinically validated in individuals with BED. Third, analyses of the relationships between changes on the SDS and other study endpoints were not conducted for this report. Fourth, high percentages of study participants were women, were white, and were overweight based on body mass index. Furthermore, individuals with comorbid illnesses and psychiatric conditions were excluded. As such, it is not known how the current findings would generalize to a more heterogeneous population. Lastly, the study only enrolled individuals with protocol-defined moderate-to-severe BED; consequently, the level of impairment in individuals with less severe BED is not known.
Conclusions
In two Phase III studies, adults with protocol-defined moderate-to-severe BED exhibited mild-to-moderate functional impairment based on mean SDS total score, mean SDS domain scores, and the mean number of days of underproductivity at baseline. A substantial proportion of study participants was also categorized as meeting criteria for nonremission based on SDS total score at baseline, which suggests that most study participants exhibited some level of functional disability. For each study, at Week 12, dose-optimized LDX (50mg or 70mg) produced significant reductions in SDS total score, SDS domain scores, and in the number of days of underproductivity while at work/school. Rates of remission versus nonremission on the SDS also favored LDX over PBO. These findings suggest that the previously reported reductions in binge-eating behavior with LDX observed in these studies6 are accompanied by reductions in functional impairment.
Acknowledgments
Under the direction of the authors, Stefan Kolata, PhD (a former employee of Complete Healthcare Communications [CHC]), and Craig Slawecki, PhD (a current employee of CHC), provided writing and formatting assistance for this manuscript. Editorial assistance in the form of proofreading, copyediting, and fact-checking was also provided by CHC.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
- Masheb RM, Grilo CM. Quality of life in patients with binge eating disorder. Eat Weight Disord. 2004;9(3):194–199.
- Kessler RC, Berglund PA, Chiu WT, et al. The prevalence and correlates of binge eating disorder in the World Health Organization World Mental Health Surveys. Biol Psychiatry. 2013;73(9):904–914.
- Sheehan KH, Sheehan DV. Assessing treatment effects in clinical trials with the discan metric of the Sheehan Disability Scale. Int Clin Psychopharmacol. 2008;23(2):70–83.
- Leon AC, Olfson M, Portera L, et al. Assessing psychiatric impairment in primary care with the Sheehan Disability Scale. Int J Psychiatry Med. 1997;27(2):93–105.
- McElroy SL, Hudson J, Ferreira-Cornwell MC, et al. Lisdexamfetamine dimesylate for adults with moderate to severe binge eating disorder: results of two pivotal phase 3 randomized controlled trials. Neuropsychopharmacology. 2015;41:1251–1260.
- McElroy SL, Hudson JI, Mitchell JE, et al. Efficacy and safety of lisdexamfetamine for treatment of adults with moderate to severe binge-eating disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72(3):235–246.
- Vyvanse®. lisdexamfetamine dimesylate. Lexington, MA: Shire US Inc.; 2017.
- Coles T, Coon C, DeMuro C, et al. Psychometric evaluation of the Sheehan Disability Scale in adult patients with attention-deficit/hyperactivity disorder. Neuropsychiatr Dis Treat. 2014;10:887–895.
- Hodgins DC. Reliability and validity of the Sheehan Disability Scale modified for pathological gambling. BMC Psychiatry. 2013;13:177.
- Arbuckle R, Frye MA, Brecher M, et al. The psychometric validation of the Sheehan Disability Scale (SDS) in patients with bipolar disorder. Psychiatry Res. 2009;165(1–2):163–174.
- Hambrick JP, Turk CL, Heimberg RG, et al. Psychometric properties of disability measures among patients with social anxiety disorder. J Anxiety Disord. 2004;18(6):825–839.
- Alaka KJ, Noble W, Montejo A, et al. Efficacy and safety of duloxetine in the treatment of older adult patients with generalized anxiety disorder: a randomized, double-blind, placebo-controlled trial. Int J Geriatr Psychiatry. 2014;29(9):978–986.
- Leon AC, Shear MK, Portera L, Klerman GL. Assessing impairment in patients with panic disorder: the Sheehan Disability Scale. Soc Psychiatry Psychiatr Epidemiol. 1992;27(2):78–82.
- Sheehan DV, Harnett-Sheehan K, Spann ME, et al. Assessing remission in major depressive disorder and generalized anxiety disorder clinical trials with the discan metric of the Sheehan disability scale. Int Clin Psychopharmacol. 2011;26(2):75–83.
- Jacobsen PL, Mahableshwarkar AR, Serenko M, et al. A randomized, double-blind, placebo-controlled study of the efficacy and safety of vortioxetine 10mg and 20mg in adults with major depressive disorder. J Clin Psychiatry. 2015;76(5):575–582.
- Weiss M, Murray C, Wasdell M, et al. A randomized controlled trial of CBT therapy for adults with ADHD with and without medication. BMC Psychiatry. 2012;12(30):1–8.
- Wilhelm S, Phillips KA, Didie E, et al. Modular cognitive-behavioral therapy for body dysmorphic disorder: a randomized controlled trial. Behav Ther. 2014;45(3):314–327.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed., text revision. Washington, DC: American Psychiatric Association; 2000.
- Sheehan DV, Harnett-Sheehan K, Raj BA. The measurement of disability. Int Clin Psychopharmacol. 1996;11(Suppl 3):89–95.
- Johnson JG, Spitzer RL, Williams JB. Health problems, impairment and illnesses associated with bulimia nervosa and binge eating disorder among primary care and obstetric gynaecology patients. Psychol Med. 2001;31(8):1455–1466.
- Kessler RC, Shahly V, Hudson JI, et al. A comparative analysis of role attainment and impairment in binge-eating disorder and bulimia nervosa: results from the WHO World Mental Health Surveys. Epidemiol Psychiatr Sci. 2014;23(1):27–41.
- Hudson JI, Hiripi E, Pope HG, Jr., Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61(3):348–358.
- Preti A, Girolamo G, Vilagut G, et al. The epidemiology of eating disorders in six European countries: results of the ESEMeD-WMH project. J Psychiatr Res. 2009;43(14):1125–1132.
- McElroy SL, Hudson JI, Capece JA, et al. Topiramate for the treatment of binge eating disorder associated with obesity: a placebo-controlled study. Biol Psychiatry. 2007;61(9):1039–1048.
- Brennan BP, Roberts JL, Fogarty KV, et al. Memantine in the treatment of binge eating disorder: an open-label, prospective trial. Int J Eat Disord. 2008;41(6):520–526.
Category: Original Research, Past Articles





