Tube Gastrostomy Management for Acute Sleeve Gastrectomy Leaks
by Jamil Luke Stetler, MD, FACS; S. Scott Davis, Jr., MD, FACS; Jahnavi K. Srinivasan, MD FACS; Arvinpal Singh, MD; Colin Johnson, PA-C; Ankit D. Patel, MD, FACS; Edward Lin, DO, MBA, FACS
Dr. Stetler is Director of Bariatrics at Emory Saint Joseph’s Hospital in Sandy Springs, Georgia. Dr. Davis is Associate Professor of Surgery and Director, Emory Endosurgery Unit for Minimally Invasive Surgery, Division of General and GI Surgery, Department of Surgery, Emory School of Medicine, Atlanta, Georgia. Dr. Srinivasan is Associate Professor of Surgery, Division of GI and General Surgery, Emory University, Atlanta, Georgia. Drs. Singh and Lin and Mr. Johnson are with Emory University Hospital, Atlanta, Georgia. Dr. Patel is Assistant Professor of Surgery, Division of General and GI Surgery, Department of Surgery, Emory School of Medicine, Atlanta, Georgia.
Abstract: Morbid obesity is an epidemic, and the number of bariatric surgeries performed each year is rising. One of the most feared complications of bariatric surgery is a leak. There are multiple modalities for treating this complication with varying efficacy, with the combination of these modalities rendering the majority (90%) of leak management nonoperative. However, some of these treatment options lack widespread availability and usually require skilled practitioners to employ. There will be times where transfer to another facility with these capabilities is not feasible. Therefore, this is an opportunity to remind management teams of using a tube gastrostomy as a treatment option for this complication. The tube gastrostomy follows the basic principles of managing deep abdominal sepsis and can be applied in most community settings.
Funding: No funding was provided for this article.
Disclosures: The authors report no conflicts of interest relevant to the content of this manuscript.
Keywords: leak, dehiscence, sleeve gastrectomy, malecot, tube gastrostomy
Bariatric Times. 2018;15(6):12–15.
Introduction
Several options have been proposed for the management of a staple line disruption following sleeve gastrectomy. These include the use of exploratory laparotomy, diagnostic laparoscopy, placement of drains, suture closure of the defect, filling the defect with a biologic plug, omental patch, use of biologic glue, clipping technology, intraluminal negative pressure therapy, endoscopic suturing, and endoscopic stenting across the defect. All of these methods have been described with varying degrees of efficacy, but the combination of these modalities have rendered the majority (90%) of leak management nonoperative.1 Another consideration in using these technologies is availability and the skills required to deploy them. Some healthcare facilities simply might not be prepared to manage this complication that occurs sporadically (1.5 to 2.2%).2,3 Moreover, devices such as an endoscopic suturing device requires technical finesse developed from repeated use. Furthermore, treating a staple line defect alone might be insufficient when there is undrained fluid or debris residing in the peritoneal cavity.
There will be occasions when the patient is unable to be transferred to another facility that can manage the staple line disruption, and the surgeon will need to have options for treatment. The principles for managing deep abdominal sepsis do not change following leaks from sleeve gastrectomy. Collections that are not amenable to antibiotics alone should be drained. Patients with peritonitis usually require surgery, and in such cases proper drain placement in sites of collections are critical. Unless immediately evident, endoscopic insufflation with air or with a gastroscope during surgery could be used to identify the site of staple line dehiscence. The management of staple line disruption is critical. The dehiscence is rarely a complete greater curvature breakdown, but rather a segment of no more than 2cm. This site presents a conundrum for the surgeon attempting to control the leak. Options often include the use of adjacent omentum or adipose tissue. Oversewing the defect with sutures can be attempted with further buttressing using biologic glue. Endoscopic procedures might also be attempted during surgery by passing a stent to cover the leak. Endoscopic suturing might not be necessary during surgery because this task can be accomplished directly by the surgeon. Drains can be placed at these sites along with nasogastric suction. A distal feeding tube may be considered for long-term nutritional support so there is less reliance on parenteral nutrition. This is an opportunity to remind the management team that directly placing a malecot drain catheter through the defect into the gastric lumen can be considered.
Technical Considerations
Drain size may be selected based on lumen size. Typically, a sleeve gastrectomy is performed using a calibration tube that is 32 to 46 French, and, therefore, the malecot catheter should be smaller (e.g., 14–20 French) to avoid complete lumen obstruction (Figure 1). The malecot catheter is usually latex (silicone equivalents are also available) and has four stabilizing wings to maintain lumen patency while permitting fluids to drain past it. One or two of these wings can be trimmed if there is desire for more rapid passage of fluids across the tube. Alternatively, a T-shaped tube can be used. Whether suture closure around the tube insertion site is required depends on the inflammation around the tissue, but we do recommend absorbable sutures to minimize foreign material interference that could delay subsequent healing. The catheter can then be brought out of either side of the abdominal wall and secured.
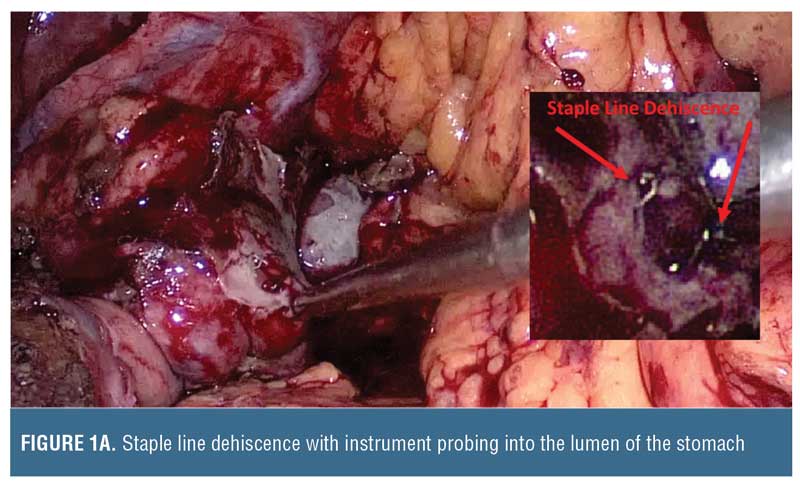
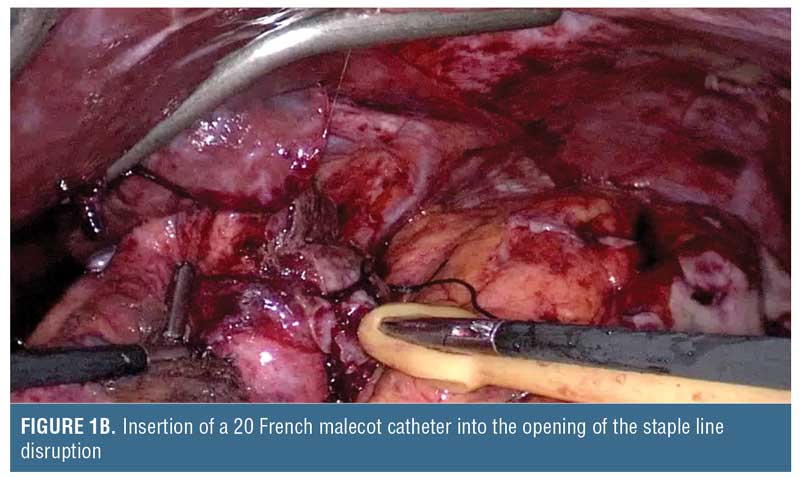
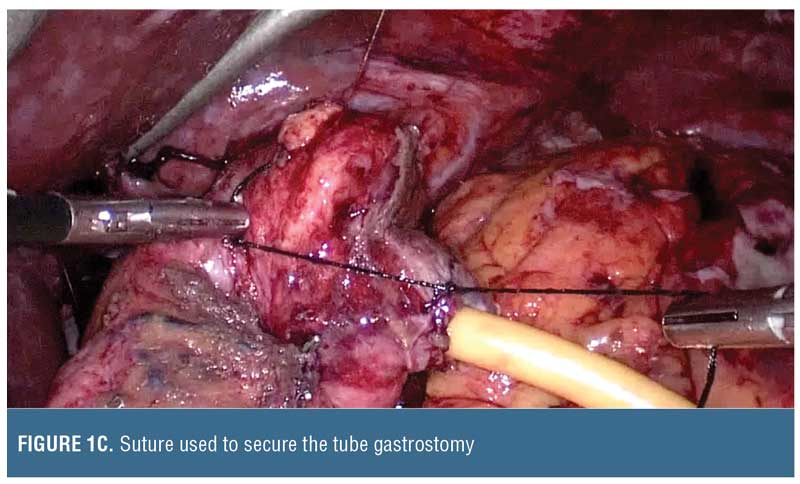
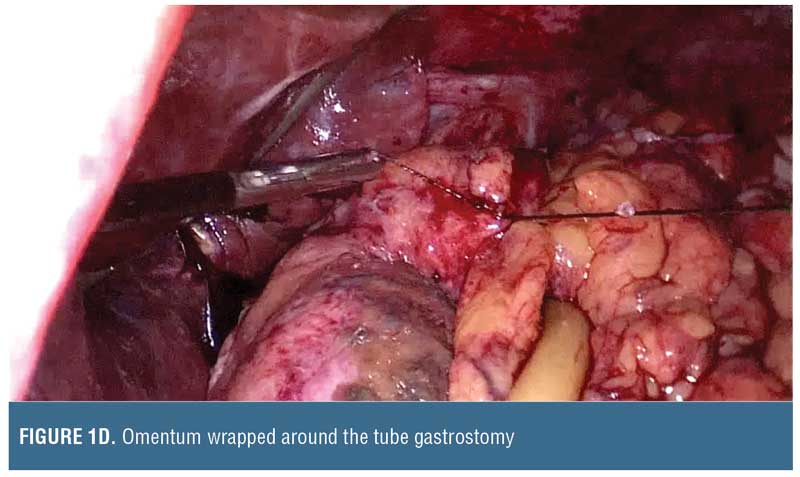
Follow-up Care
Placing such a drain through the leak site is not a novel concept. In essence, a controlled gastrocutaneous fistula is created, allowing the patient to recover from the intra-abdominal sepsis. After 1 to 2 weeks, a water-soluble oral contrast study may be performed to determine if the leak is controlled (i.e., no extravasation) prior to commencing oral liquids (Figure 2). Oral intake containing solid particles will likely cause mechanical obstruction and is not recommended. After the patient is able to tolerate clear liquids for a few days, the external end of the catheter can be clamped off and used to vent as needed, but the patient should continue to take clear liquids. If the patient continues to tolerate oral liquids, and there is no distal gastric obstruction (evaluated by upper gastrointestinal [UGI] or endoscopy) that could potentially cause back pressure blowout of the leak site or cause a persistent fistula, the catheter may be withdrawn 3 to 4cm, converting this intraluminal drain to an external drainage catheter. Of note, since the intraluminal drain is not a Stamm tube, the surgeon needs to allow approximately 3 to 4 weeks for a tract to form around the drain prior to backing it out. Then, every 3 to 4 days, the catheter should be withdrawn 3 to 4cm until it is completely exteriorized and discarded. There are no firm rules regarding how rapid these drains should be backed out, and similarly there are no rules on how often contrast studies need to be performed during the drain withdrawal period. Once the drain is removed, it can take 5 to 7 days for the fistula tract to seal up completely.
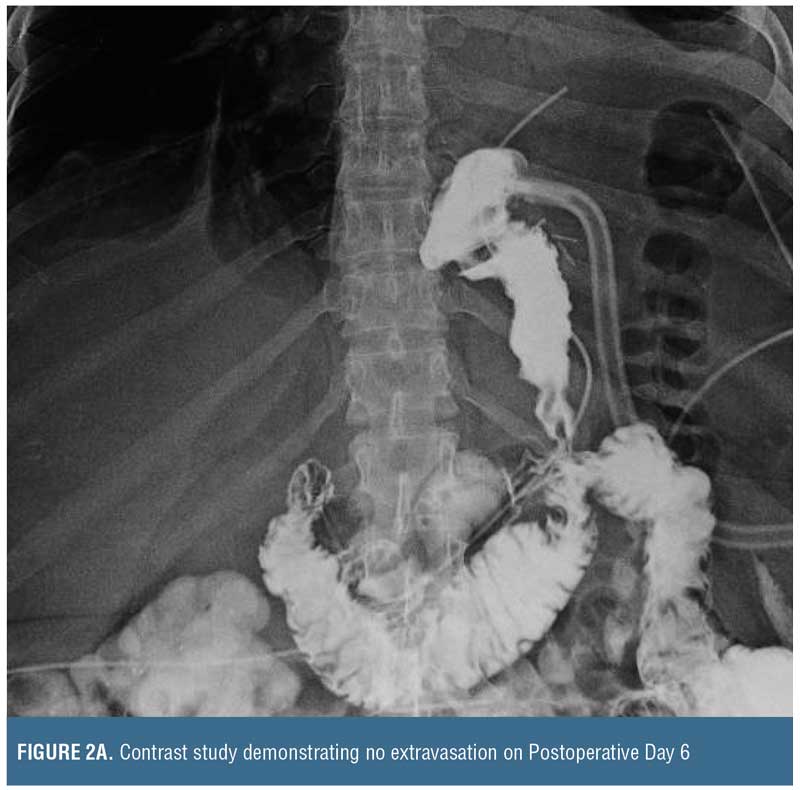
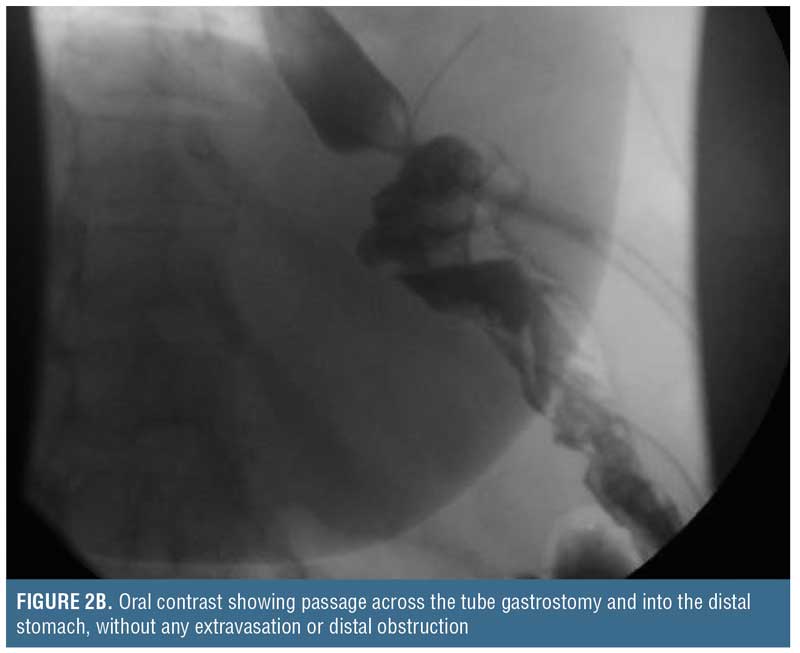
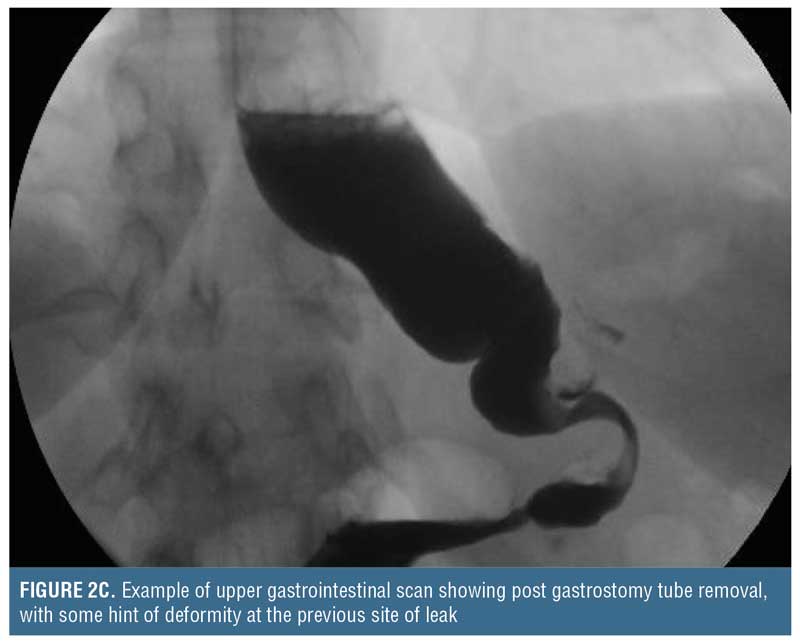
Finally, if there is contrast extravasation on the initial follow-up study, it should not be considered treatment failure because the catheter allows direct percutaneous drainage. In this situation, the surgeon should be mindful of the factors that impact fistula closure (i.e., FRIEND mnemonic: foreign body, radiation, inflammation/infection/inflammatory bowel disease, epithelialization, neoplasm, and distal obstruction). Once addressed, if a chronic fistula persists, then the patient may be treated with any of the previously named adjuncts, including nutritional support to promote recovery, prior to repeating a contrast study.
Discussion
Placing drains directly into a leak is not novel. Surgeons have used such techniques to convert a freely leaking viscus into a controlled fistula in acutely ill patients for a long time. It has been used for duodenal stump blowouts as well as esophageal perforation.4 While most sleeve gastrectomy leaks are treated without surgery, approximately 10 percent of patients who experience leaks require surgical intervention, and it is in such patients that converting a lumen into a controlled fistula by way of direct drain placement should be considered.
Many modalities are available in the management of the acute leaks with varying degrees of success (Table 1). In the acute setting with inflammation, suturing techniques are precarious because inflamed tissues tend not to close. It is entirely feasible to patch with free omentum, but the high-pressure zones within the gastric lumen could increase blowouts of the patch.5 It might be more prudent to place a tube gastrostomy and patch over the site with omentum.
Endoscopic suturing or closing devices require air or CO2 insufflation, which could further exacerbate abdominal contamination in the acute setting. In our experience, placing a large clip over the hole typically works for small disruptions but can later render the site inoperable because of the large metallic object. We have used biologic plugs and glues with unpredictable success because of migration or inadequate placement. We have even used endoscopic snares to retrieve omentum and draw it into the lumen as a natural plug.
In acute leaks (first 3 weeks), stents are popular management modalities due to their relative ease of application and the notion that they “cover” a hole. Stent placement could reduce the volume of leakage but rarely completely seals it; there is frequently leakage between the wall of the stent and the gastric lumen. Stent migration is common, and additional stents might be required to fully cross the area of leak. Most stents require removal or exchange within 3 to 6 weeks. The gastric lumen after stent removal is often inflamed, making it difficult to identify and characterize the hole. The stent should be long enough to cover the site of leak. The diameter of the stent should be wide enough to cover the lumen with minimal migration but not so big that it stresses the staple line dehiscence.
The modality for managing the acute sleeve gastrectomy staple line dehiscence will depend on what is available and the skills of the available surgeon. In the patient who requires surgery, a tube gastrostomy has advantages. In our experience, closure might be achieved in four weeks. In contrast, the use of other modalities requires multiple and repetitive interventions that can take several months to achieve closure.
Lastly, we have encountered delayed recurrence of leaks after they were managed with different nonoperative techniques: one occurred six years after treatment and another after nine years. The recurrences appeared to be due to weakening of the tissue closure. In both patients, surgery was required at the second presentation.
In summary, this is a reminder to the surgeon that a tube gastrostomy is a viable and time-tested modality that can be applied to acute sleeve gastrectomy staple line dehiscence, especially if access to other treatment modalities is limited.
References
- Aurora A, Khaitan L, Saber A. Meta-analysis of leak after laparoscopic sleeve gastrectomy for morbid obesity. Presented at SAGES Scientific Meeting; 11–13 Apr 2018; Seattle, Washington.
- Sakran N, Goitein D, Raziel A, et al. Gastric leaks after sleeve gastrectomy: a multicenter experience with 2,834 patients. Surg Endosc. 2013;27(1):240–245.
- Parikh M, Issa R, McCrillis A, et al. Surgical strategies that may decrease leak after laparoscopic sleeve gastrectomy: a systematic review and meta-analysis of 9991 cases. Ann Surg. 2013;257:231–237.
- Ojima H, Kuwano H, Sasaki S, et al. Successful late management of spontaneous esophageal rupture using T-tube mediastinoabdominal drainage. Am J Surg. 2001;182: 192–196.
- Galal KI, Osama HT, Ahmed R, Amr M. The effects of adding gastrojejunostomy to sleeve gastrectomy on GERD, food tolerance, and weight loss: a randomized study. Bariatric Surgical Practice and Patient Care. 2017:107–115.
- Kim J, Azagury D, Eisenberg D, et al. ASMBS position statement on prevention, detection, and treatment of gastrointestinal leak after gastric bypass and sleeve gastrectomy, including the roles of imaging, surgical exploration, and nonoperative management. Surg Obes Relat Dis. 2015;11(4):739–748.
- Donatelli G, Ferretti S, Vergeau BM, et al. Endoscopic internal drainage with enteral nutrition (EDEN) for treatment of leaks following sleeve gastrectomy. Obes Surg. 2014;24(8):1400–1407.
- Shoar S, Poliakin L, Khorgami Z, et al. Efficacy and safety of the over-the-scope clip (OTSC) system in the management of leak and fistula after laparoscopic sleeve gastrectomy: a systematic review. Obes Surg. 2017;27(9):2410–2418.
- El Hassan E, Mohamed A, Ibrahim M, et al. Single-stage operative management of laparoscopic sleeve gastrectomy leaks without endoscopic stent placement. Obes Surg. 2013;23(5):722–726.
- Csendes A, Braghetto, Leon P, et al. Management of leaks after laparoscopic sleeve gastrectomy in patients with obesity. J Gastrointest Surg. 2010;14(9):1343–1348.
- Corona M, Zini C, Allegritti M, et al. Minimally invasive treatment of gastric leak after sleeve gastrectomy. Radiol Med. 2013;118(6):962–970.
- Leeds SG, Burdick JS. Management of gastric leaks after sleeve gastrectomy with endoluminal vacuum (E-Vac) therapy. Surg Obes Relat Dis. 2016;12(7):1278–1285.
Category: Past Articles, Review







