Glucagon…More Than a GI Hormone for Laparoscopic Sleeve Gastrectomy

by Brandon M. Smith, MD; Logan T. Mellert, DO; Ashley Shoemaker, MA; and Adrian G. Dan, MD, FACS, FASMBS
Drs. Smith, Mellert, and Dan and Ms. Shoemaker are with the Department of Surgery, Akron City Hospital Summa Health in Akron, Ohio, and Northeast Ohio Medical University in Rootstown, Ohio.
Funding: No funding was provided for this article.
Disclosures: The authors have no conflicts of interest relevant to this article.
Bariatric Times. 2022;19(3):16–18.
Abstract
Background: The laparoscopic sleeve gastrectomy (LSG) is the most commonly performed metabolic and bariatric surgery (MBS) worldwide. Staple line leak is one of its most feared complications, and significant effort has been undertaken to identify strategies to mitigate this risk. Administration of intravenous (IV) glucagon has a well-described physiologic effect of inducing gastric atony. The potential use of glucagon prior to stapling in sleeve gastrectomy to mitigate risk of staple line leak has not been explored.
Methods: A literature review was written describing the effects of glucagon on gastrointestinal (GI) smooth muscle and its therapeutic application. The surgical challenges of sleeve gastrectomy are also reviewed, and the rationale of utilizing glucagon to address these challenges is outlined. A retrospective review of a single institution database was performed, and data was analyzed.
Results: A total of 93 primary sleeve gastrectomies were performed with routine administration of IV glucagon 2mg. The group had a female-to-male ratio of 60:33, mean age of 50.0 years, and mean body mass index (BMI) of 59.4kg/m2. There was a reoperation rate of 2.15 percent (2/93: 1 recurrent hiatal hernia with sleeve obstruction and 1 missed iatrogenic esophageal bougie injury). There was a 30-day readmission rate of 2.15 percent (2/93 for bleeding). The rates of staple line leak and stricture/obstruction were both zero percent. There were no adverse effects related to glucagon administration.
Conclusion: The effects of glucagon on intestinal smooth muscle have been well described. Its use in sleeve gastrectomy to induce gastric atony prior to stapling has sound conceptual foundation. This article reflects a single surgeon’s experience with the use of glucagon to improve the uniform creation of the sleeve gastrectomy conduit and decrease perioperative complications.
Ketwords: Laparoscopic sleeve gastrectomy, glucagon, bariatric surgery complications
In the words of hall-of-fame football coach Vince Lombardi, “Perfection is not attainable, but if we chase perfection, we can catch excellence.” The refinement of techniques in metabolic and bariatric surgery (MBS) has exemplified the words of Coach Lombardi through persistent innovation since the first operations for weight loss were reported in the 1950s. Numerous surgical procedures were devised to combat obesity over the subsequent decades, and each new procedure was designed to build upon the advantages or overcome the drawbacks of the previous operations. In the late 1990s, Dr. Michel Gagner proposed performing the biliopancreatic diversion with duodenal switch (BPD-DS) as a staged operation, beginning with the laparoscopic sleeve gastrectomy (LSG).1 The highly favorable outcomes following the initial LSG prompted its emergence as a stand-alone procedure. Today, the LSG has become the most commonly performed metabolic operation in the United States (US), comprising 67 percent of metabolic and bariatric procedures in 2018.2
The Challenge of Gastric Spasms
The popularity of the LSG is a result of its excellent safety profile, reproducible outcomes, and technical simplicity when compared to diversional procedures. When complications do occur, however, the LSG can be unforgiving, particularly with regard to staple line leaks and obstructions at the incisura angularis. Staple line disruptions remain the most feared complication for surgeons, with an incidence of approximately 1.5 percent.3 Considerable innovation and research efforts have been focused on developing strategies to mitigate the risk of leaks. These include surgeon education, refinement of operative technique,4 determining ideal bougie size,5 optimizing distance from the pylorus,4 and the use of staple line reinforcement.3 In addition, the selection of appropriate staple line height for the given target tissue is critical for achieving an optimal tissue compression and seal. As the stomach is a muscular organ with three distinct layers (outer longitudinal, middle circular, and inner oblique muscles), any spasm can alter tissue thickness significantly. The spasms themselves might occur due to stomach manipulation, previous staple line tissue transection/trauma, tissue irritation from cold carbon dioxide, tissue desiccation from direct insufflation, or a plethora of other mechanical or physiologic stimuli. Therefore, choosing the staple height appropriate for the target tissue is of paramount importance, and any opportunity to reduce gastric tissue variability for stapling should be investigated and explored. Undoubtedly, there are countless variations and methods for performing LSG, and herein, we advocate for the consideration of glucagon administration to reduce gastric smooth muscle spasms prior to stapling.
Glucagon—Physiologic Effects and Clinical Utility
 Glucagon, a pancreatic hormone best known for its insulin-opposing role in systemic glucose homeostasis, also has a powerful induction effect on the relaxation of gastrointestinal (GI) smooth muscle (Figure 1). The use of glucagon to temporarily alter the dynamic motility of the smooth muscle in the gastrointestinal tract is not a novel idea. As early as 1969, Dr. Ann Lawrence from the University of Chicago published articles on the impact of glucagon, highlighting its physiologic effects of transient marked bowel relaxation after systemic administration.6,7 Subsequently, Miller et al8 quantified the dose-response relationship in the late 1970s. Through intravenous administration of glucagon at doses of 0.25, 0.5, 1.0, or 2.0mg, they found the onset of decreased gastrointestinal motility and decreased smooth muscle tonicity occurred approximately 45 seconds after administration, regardless of dose. Furthermore, the duration of the drug’s effect was proportional to the dose quantity.8 Following 2mg of intravenous (IV) glucagon, stomach atony persisted for 15 minutes, with an additional seven minutes of hypotonicity8 (roughly the time needed to perform the vertical stapling for LSG). The work of Miller and colleagues centered largely on GI radiography, namely hypotonic-duodenography and barium enema imaging, and they consistently demonstrated attainment of improved GI radiographic image quality and diagnostic ability with glucagon administration prior to imaging.
Glucagon, a pancreatic hormone best known for its insulin-opposing role in systemic glucose homeostasis, also has a powerful induction effect on the relaxation of gastrointestinal (GI) smooth muscle (Figure 1). The use of glucagon to temporarily alter the dynamic motility of the smooth muscle in the gastrointestinal tract is not a novel idea. As early as 1969, Dr. Ann Lawrence from the University of Chicago published articles on the impact of glucagon, highlighting its physiologic effects of transient marked bowel relaxation after systemic administration.6,7 Subsequently, Miller et al8 quantified the dose-response relationship in the late 1970s. Through intravenous administration of glucagon at doses of 0.25, 0.5, 1.0, or 2.0mg, they found the onset of decreased gastrointestinal motility and decreased smooth muscle tonicity occurred approximately 45 seconds after administration, regardless of dose. Furthermore, the duration of the drug’s effect was proportional to the dose quantity.8 Following 2mg of intravenous (IV) glucagon, stomach atony persisted for 15 minutes, with an additional seven minutes of hypotonicity8 (roughly the time needed to perform the vertical stapling for LSG). The work of Miller and colleagues centered largely on GI radiography, namely hypotonic-duodenography and barium enema imaging, and they consistently demonstrated attainment of improved GI radiographic image quality and diagnostic ability with glucagon administration prior to imaging.
Inducing GI atony offers further benefits beyond simple tissue manipulation. Glucagon has been previously employed to optimize tissue and provide uniform compression in preparation for intestinal stapling. In 1979, Dr. Francis Harford utilized the drug for end-to-end anastomosis (EEA) stapling in colorectal surgery. He had also reported difficulty and occasional unsuccessful passage of the EEA device through the rectum due to rectal stump muscle spasm. After administration of 2mg of glucagon, Dr. Harford appreciated adequate relaxation of the rectal stump, allowing passage of the EEA stapler, and published this technique for the selective use of glucagon for coloproctostomy.9
Utilization of glucagon to provide smooth muscle relaxation was subsequently applied to foregut operations. In 1994 at the University of Nebraska, Robinson et al10 used 1mg of IV glucagon prior to performing circular stapled esophago-gastric anastomoses. They reported findings in accordance with those of Miller et al two decades earlier. They described an onset of action of 45 seconds, with esophageal and gastric smooth muscle relaxation persisting for 20 to 30 minutes. This physiologic effect allowed the stomach to stretch more easily to reach higher within the thoracic cavity.
Arguably, the most common and well-known surgical use of glucagon is induction of sphincter of Oddi hypotonicity in the setting of intraoperative cholangiography. Cannon and Legge11 recorded their observed effects of glucagon on 35 cholangiography studies in 1979, noting improved anatomic detail and diagnostic accuracy in 28 of 35 patients. Reproducible findings of sphincter of Oddi relaxation after glucagon administration have led to its frequent clinical use in the management of choledocholithiasis. Surgical algorithms for the management of choledocholithiasis involve intraoperative cholangiography coupled with glucagon administration and attempted antegrade flushing of the stones into the duodenum.12 Administration of glucagon has been shown to be safe and effective in the management of commonly encountered bile duct pathology, as there is no evidence of common adverse events.12
In 2019, a single institution retrospective study was presented at the American College of Surgeons Clinical Congress attempting to further explore a potential quantifiable clinical benefit in the application of glucagon to foregut procedures. Dr. Hiroyuki Kobayashi from Japan, concerned about gastroesophageal anastomotic leaks, set out to answer two questions: 1) Can glucagon injection increase gastric tube length? and 2) Can the elongated gastric tube reduce the rate of anastomotic leak? He reported his data on 53 esophagostomies with gastric pull-up reconstruction with 1mg glucagon administration. Glucagon resulted in a 2.8±2.0cm elongation of the gastric tube conduit and a significant 7.2 percent decrease in anastomotic leakage. The proposed mechanism was that glucagon-induced elongation allowed for reach and creation of the anastomosis at a site with better blood flow.13
The physiologic principle of administering glucagon to transiently alter dynamic anatomy is well established by several historical accounts of its use, as outlined above. To our knowledge, however, there are no data yet to support the clinical utility of glucagon to optimize gastric tissue thickness in metabolic and bariatric surgical procedures.
Application to Surgical Technique in Sleeve Gastrectomy
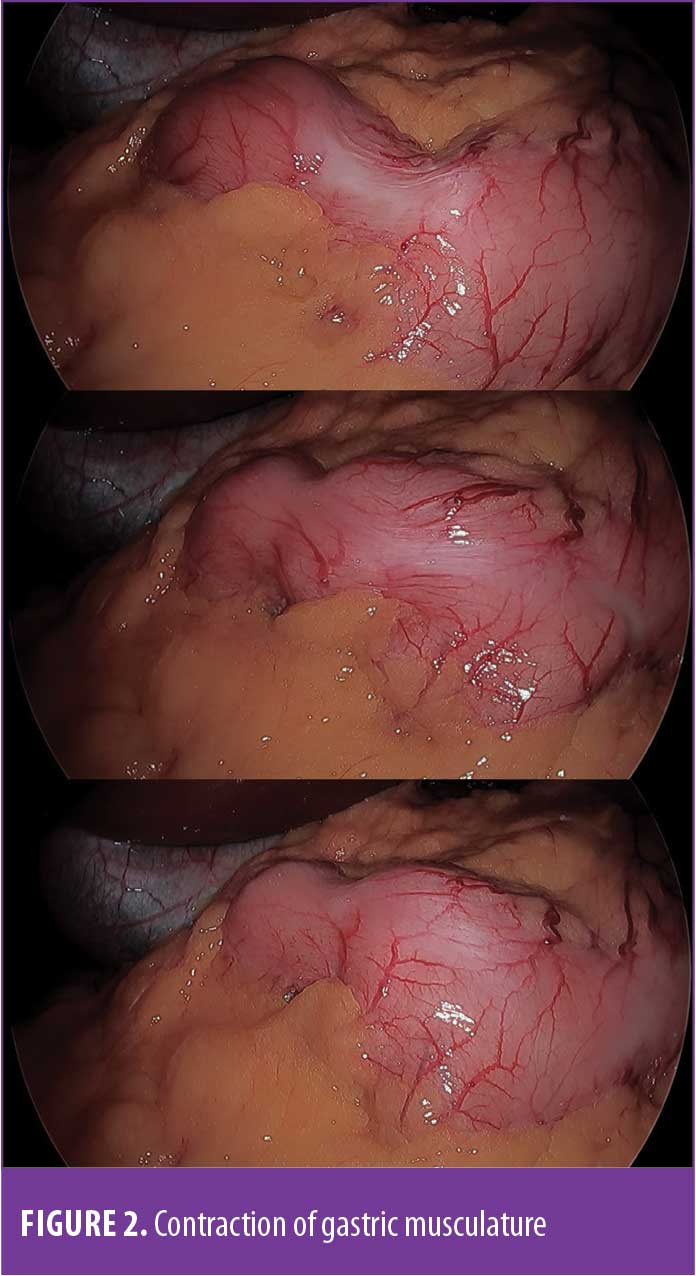
Since the initial reports of LSG as the first part of BPD-DS, the procedure has undergone technical modifications to improve outcomes and decrease the variability of the operation among centers and surgeons. This has led to the refinement of the surgical technique, including utilization of appropriate size calibration bougies5,14 and commercially available devices to calibrate the diameter of the conduit.15 Staple line reinforcement products have also been employed to bolster the strength of the staple line and reduce leak rate.16 The surgeon’s choice of the various available staple heights, however, remains one of the most important factors in achieving an intact and robust staple line with ideal mechanical staple formation. When the first firing of the staple line is performed (usually 2–4cm from the pylorus and nearly parallel to the lesser curvature), a variable, yet sometimes powerful, contraction of the smooth muscle layers of the distal stomach might be elicited (Figure 2). This has been anecdotally encountered with transection and manipulation of the thick antrum but is rarely observed on the less muscular proximal stomach, such as during construction of the gastric pouch in laparoscopic Roux-en-Y gastric bypass.
Glucagon is a safe, well-tolerated, and commonly used medication. The most common side effect is nausea, but in the setting of abdominal surgery, this is of negligible concern, as the patient is anesthetized. There are few contraindications to glucagon administration. Most notably, glucagon should be avoided in patients with pheochromocytoma, as glucagon can induce a catecholamine surge.17 It should also be avoided in patients with insulinoma, as glucagon has been described to induce insulinoma stimulation, leading to critical hypoglycemia.17 A secondary consideration is the financial cost of glucagon. With no generic alternative available in the US, the cost of glucagon has a listed retail price of $280.80, although this price is variable and may differ depending on formulary contracts and bulk purchases.18
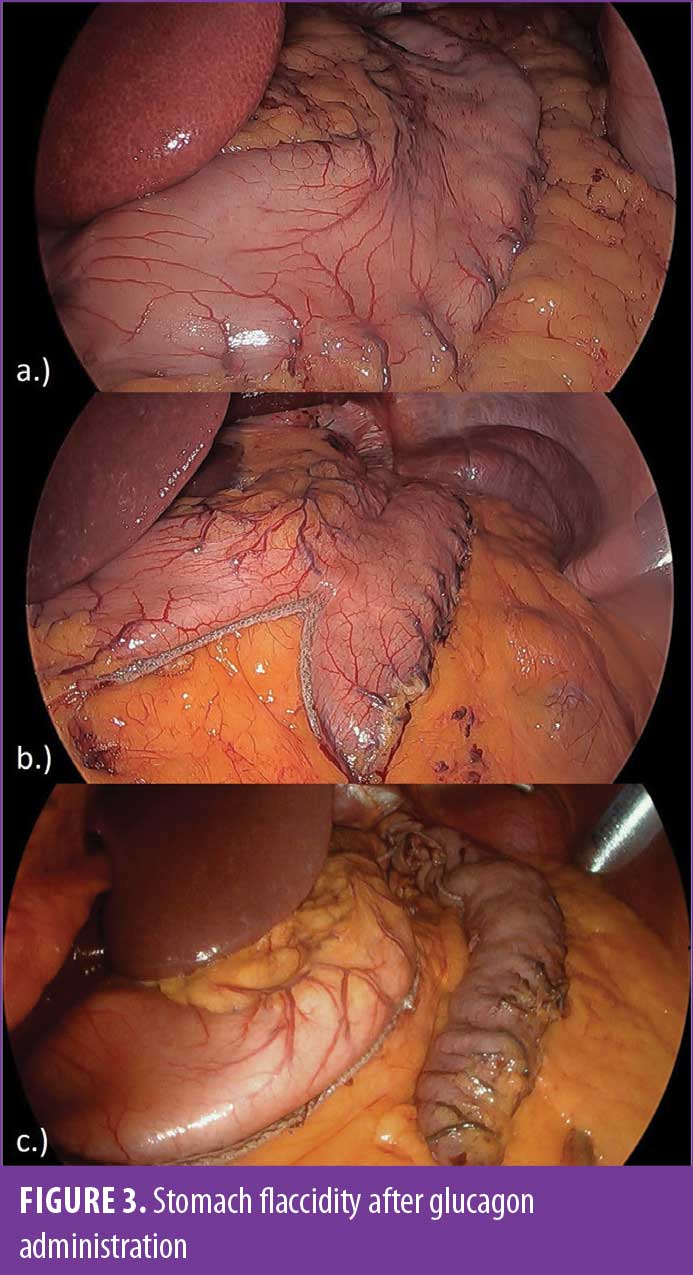
Because of the occasional occurrences of severe distal gastric spasms, we have integrated the utilization of glucagon in our LSG technique. Over the past two years, with routine use by one surgeon, this has nearly eliminated clinically significant gastric spasms and contractions (Figure 3). We now administer 2mg of glucagon intravenously after completion of our gastric mobilization and just prior to commencing gastric stapling. We have found a consistent and reproducible relaxation of the stomach secondary to the antispasmodic properties of glucagon. The flaccid stomach drapes over the bougie with a loose hug, allowing for improved visualization of the bougie outline within the stomach (Figure 3). In the setting of gastric atony, the bougie contour maintains this outlined position, allowing us to align the laparoscopic linear stapling device in the desired firing position with minimal stomach irregularities. We have noticed a more uniform sleeve gastrectomy and a decrease in unintentional spiraling of the conduit, a well-described cause of sleeve conduit stenosis.19 Additionally, the known cardiovascular potentiating effects of glucagon result in increased splanchnic blood flow.20 Theoretically, an increase in gastric blood flow would facilitate the detection of vessels, which could appear hemostatic due to spasms and would be at risk of bleeding in the immediate postoperative period when vessels relax.
As the thickness of the stomach is variable, many surgeons alter their staple height as they progress proximally from the antrum to the thinner fundus while shaping the sleeve conduit. The goal of this technique is to achieve a uniform tissue approximation with appropriate staple height along the entirety of the staple line. This strategy, however, does have an inherent transition in compression depth when staple loads of various heights intersect. Many surgeons address this potential weakness by placing staple line reinforcement sutures at intersecting staple lines. In addition to altering staple heights, our experience with glucagon induced gastric atony has led us to appreciate a more uniform approximation within each staple load, reducing interload compression variability and yielding a more uniformly constructed sleeve conduit.
We have utilized glucagon selectively in situations where muscle spasms occurred during the creation of the staple line for nearly a decade. Over the past two years, however, one surgeon has employed glucagon routinely just after the complete mobilization of the stomach and prior to firing the first staple load. Stapling was performed starting 4cm proximal to the pylorus and progressing toward the angle of His. The size of the conduit was calibrated over a 40-French bougie. During this period, a total of 93 primary LSGs were performed with routine administration of IV glucagon 2mg. Revisional operations and LSGs involving the takedown of previous fundoplication were excluded. The group had a female-to-male ratio of 60:33, an average age of 50.0 years, and an average body mass index (BMI) of 59.4kg/m2. This retrospective review of our institutional database shows a reoperation rate of 2.15 percent (2/93: 1 recurrent hiatal hernia with sleeve obstruction and 1 for a missed iatrogenic esophageal bougie injury in a patient who underwent a concomitant large hiatal hernia repair) and a 30-day readmission rate of 2.15 percent (2/93 for bleeding). The rates of staple line leak and stricture/obstruction were both zero percent. No adverse effects related to the administration of glucagon were recorded. This data is on par with acceptable outcomes reported in the literature.
Limitations. Evident weaknesses of our study are the limited data, the relatively small single surgeon sample size, the lack of a control group, and its retrospective nature. The lack of a control group and the low complication rates associated with LSG make it difficult to draw any conclusions regarding the benefits of glucagon beyond the anecdotal experience that gastric smooth muscle spasms are reduced, thus helping reduce potential stapling-related technical problems. In our practice, we would also find it difficult to omit this useful adjunct if it were needed in a patient randomized to the control group. The subject does not lend itself well to small animal studies because the thickness of the human stomach varies significantly from that of small animals.
Conclusion
The effects of glucagon on intestinal smooth muscle have been well described. Its utilization in LSG was attempted out of necessity for cases where an overt smooth muscle spastic reaction occurred with tissue manipulation. This article reflects a single surgeon’s experience with the use of glucagon to improve the uniform creation of the LSG conduit and decrease perioperative complications. Given the current standardization of the LSG technique and the very low incidence of staple line leak, an adequately powered study would require a larger patient sample size to detect a statistically significant clinical benefit of glucagon utilization. The potential value of glucagon warrants further exploration through larger prospective, randomized, multi-center trials seeking to obtain higher quality evidence to quantify its clinical benefits.
References
- Gagner M, et al. Laparoscopic sleeve gastrectomy with second stage biliopancreatic diversion and duodenal switch in the superobese. In: DeMaria EJ, Inabnet WB, Ikramuddin S, eds. Laparoscopic Bariatric Surgery. Lippincott Williams & Wilkins; 2005:143–150.
- English WJ, DeMaria EJ, Brethauer SA, et al. American Society for Metabolic and Bariatric Surgery estimation of metabolic and bariatric procedures performed in the United States in 2016. Surg Obes Relat Dis. 2018;(3):259–263.
- Ganer M, Kemmeter P. Comparison of laparoscopic sleeve gastrectomy leak rates in five staple-line reinforcement options: a systematic review. Surg Endosc. 2020;34(1):396–407.
- Parikh M, Issa R, McCrillis A, et al. Surgical strategies that may decrease leak after laparoscopic sleeve gastrectomy. Ann Surg. 2013;257(2):231–237.
- Parikh M, Gagner M, Heacock L, et al. Laparoscopic sleeve gastrectomy: does bougie size affect mean %EWL? Short-term outcomes. Surg Obs Relat Dis. 2008;4(4):528–533.
- Lawrence AM. Glucagon. Annu Rev Med. 1969;20:207–222.
- Lawrence AM. Glucagon in medicine: new ideas for old hormone. Med Clin North Am. 1970;54:183–190.
- Miller RE, Chernish SM, Brunelle RL, Rosenak BD. Double blinded radiographic study of dose response to intravenous glucagon for hypotonic duodenography. Radiology. 1978;127:55–59.
- Hardord FJ. Use of glucagon in conjunction with the end-to-end anastomosis (EEA) stapling device for low anterior anastomoses. Dis Colon Rectum. 1979;7:452–454.
- Robinson LA, Moulton AL, Fleming WH. Techniques to simplify esophagogastric circular stapled anastomoses. J Surg Oncol. 1994;57:266–269.
- Cannon P, Legge D. Glucagon as a hypotonic agent in cholangiography. Clin Radiol. 1979;30:49–52.
- Lilly MC, Arregui ME. A balanced approach to choledocholithiasis. Surg Endosc. 2001;15:467–472.
- Kobayashi H. Impact of intravenous injection of glucagon on anastomotic leakage in esophagectomy. J Am Coll Surg. 2019;229(4):E116.
- Palermo M, Serra E. Laparoscopic sleeve gastrectomy: how do I do it. Laparoendosc Adv Surg Tech. 2020;30(1):2–5.
- Nandra K, Ing R. Safety of orogastric tubes in foregut and bariatric surgery. Surg Endosc. 2018;32:4068–4070.
- Gagner M, Buchwald J. Comparison of laparoscopic sleeve gastrectomy leak rates in four staple-line reinforcement options: a systematic review. Surg Obs Relat Dis. 2014;10(4):713–723.
- Miller R, Chernish S, Greenman G, et al. Gastrointestinal response to minute doses of glucagon. Radiology. 1982;143(2):317–320.
- Eli Lilly. Glucagon cost with or without insurance: glucagon™ (glucagon for injection) https://www.lillypricinginfo.com/glucagon. Accessed May 2021.
- Nath A, Yewale S, Tran T, et al. Dysphagia after vertical sleeve gastrectomy: evaluation of risk factors and assessment of endoscopic intervention. World J Gastroenterol. 2016;22(47):10371–10379.
- Kock NG, Tibblin S, Schenk WG. Hemodynamic responses to glucagon: an experimental study of central, visceral and peripheral effects. Ann Surg. 1970;171(3):373–379.
Paired Editorial: Comment on “Glucagon…More Than a GI Hormone for Laparoscopic Sleeve Gastrectomy”
by Ann Rogers, MD, FACS, FASMBS
Dr. Rogers is with Penn State Health, Professor, Penn State College of Medicine, Division of Minimally Invasive and Bariatric Surgery, Hershey, PA
Funding: No funding was provided
Disclosures: The author has no conflicts of interest relevant to the content of this article.
Bariatric Times. 2022;19(3):17.
Smith et al present a novel protocol to make sleeve gastrectomy a more uniform procedure, given the limitations of current laparoscopic and robotic stapling technology. The group describes the injection of glucagon intraoperatively to induce gastric hypotonicity and decrease or eliminate muscular contractions that might randomly contribute to differential tissue thickness during the stapling process.
Surgeons must choose staple load height an average of 4 to 7 times during a typical sleeve gastrectomy.1 One technological limitation is in fixed staple height; staples do not currently fashion their ultimate closure height around tissue thickness. As such, most surgeons choose staple heights based on what they learned in training, typical tissue thickness, the presence of scar tissue, industry recommendations, and other factors. The optimal staple height would compress tissue sufficiently to allow for hemostasis and impermeability, while not crushing tissue to the point of ischemia. The stomach is thickest in the antrum and generally becomes progressively thinner moving vertically toward the fundus. Hence, most surgeons use taller staples in the antrum and less tall staples in the fundus, although this is not universal.2 The difficulty lies in predicting when one patient’s tissue will be thicker than usual and whether this calls for a change in technique.
One approach to this question lies in stapler technology algorithms that give feedback to surgeons that tissue is thicker than was “expected” by the stapler. Certainly there is no linear stapler technology yet that tells a surgeon that tissue is thinner than expected, and to therefore downsize the load;3 such technology would likely lead to a decrease in staple line bleeding. Huang and Gagner4 agreed that a device to directly measure gastric wall thickness (GWT) would be quite useful. The literature is scant. In a study of 50 resected sleeve specimens, Rawlins et al5 demonstrated that male individuals have a thicker antrum than female individuals and that increasing body mass index (BMI) significantly increased GWT, but only in patients with a BMI over 50kg/m2. Lee et al’s study of 30 sleeve specimens showed that at least at the midbody of the stomach (not antrum or fundus), there was a significant association between certain patient factors and increased GWT; these included advanced age, male sex, diabetes, and smoking history.1 This at least could be a general guide in such patients to possibly choose a taller load in the middle part of the staple line.
Regarding Smith’s protocol, glucagon has long been used by radiologists to reduce visceral contractions and aid in visualization of tissues during contrast studies.6 However, glucagon has been shown to significantly increase gastric mucus production, decrease lower esophageal sphincter pressure, and increase spontaneous gastroesophageal reflux.7 How this would impact a patient under or recovering from anesthesia for gastric surgery is not yet known. The authors correctly state that larger trials, preferably randomized and controlled, are needed. This would help illuminate whether, for example, later recurrent spasm might lead to bleeding or leakage in a given patient. At this time, much remains unknown in sleeve surgery and further clarification is always welcome.
References
- Lee YJ, Kim YN, Park S. Measurement of stomach wall thickness to guide staple selection during sleeve gastrectomy. Obes Surg. 2020. 30;2140–2146.
- Woodman GE, Voeller GR. Sleeve gastrectomy performed with single staple height and bioabsorbable reinforcement in a single surgeon >2500 consecutive case series: is smart technology necessary? Obes Surg. 2022.
- Chekan E, Whelan RL. Surgical stapling device-tissue interactions: what surgeons need to know to improve patient outcomes. Med Devices. 2014;9(7):305–318.
- Huang R, Gagner M. A thickness calibration device is needed to determine staple height and avoid leaks in laparoscopic sleeve gastrectomy. Obes Surg. 2015; 12(25):2360–2367.
- Rawlins L, Rawlins M, Teel D. Human tissue thickness measurements from excised sleeve gastrectomy specimens. Surg Endosc. 2014;28:811–814.
- Chernish SM, Skucas J, Rosenak BD, et al. Hypotonic roentgenography with glucagon. Am J Roentgenol. 1974;121(2):264–274.
- Feczko PJ, Simms SM, Iorio J, et al. Gastroduodenal response to low-dose glucagon. Am J Roentgenol. 1983;140:935–940.
Category: Past Articles, Review



