Hemostasis Techniques in Bariatric Surgery
 by Frank Villa, MD, and Bipan Chand, MD, MBA
by Frank Villa, MD, and Bipan Chand, MD, MBA
Drs. Villa and Chand are with the Department of Surgery, Loyola University Medical Center in Maywood, Illinois.
Funding: No funding was provided.
Disclosures: The authors have no conflicts of interest relevant to the content of this article.
Abstract: Bariatric surgery is a well-established means of effective and sustained weight loss. Although the overall complication rates are low, one of the most common complications following surgery is postoperative bleeding. Given the altered anatomy in patients following bariatric surgery, it is not only important to understand the approach to developing a differential diagnosis, but also to their workup and management if bleeding is suspected. Recent advancements have provided clinicians with a wider scope of options, such as endoscopic techniques, to manage and treat this potentially catastrophic complication. Also, being cognizant of the epidemiology and risk factors for postoperative bleeding bariatric surgery patients provides a direction for the development of preventative techniques.
Keywords: Bariatric surgery, early postoperative bleeding, late postoperative bleeding, intraluminal bleeding, extraluminal bleeding, melena, hematochezia, hematemesis, hemostasis, endoscopic techniques
Bariatric Times. 2020;17(2):16–18.
Obesity, defined as a body mass index (BMI) greater than 30kg/m2, has been increasing in prevalence for over a decade.1 Affecting more than 300 million people and approximately 1 in 3 American adults (36.5%), obesity has become a worldwide epidemic.1–4 The significance of this disease is related to its associated comorbidities, such as the prevalence of metabolic syndrome (39.2%) and diabetes (14.2%), in this population.2 Furthermore, a recent study on the association between different grades of obesity and the number of life-years lost indicated that life expectancy can shortened by as much as 20 years in individuals with severe obesity.5
While lifestyle modification and pharmacologic therapy for weight loss offer some success, the potential for significant and sustained weight loss is limited.2 In contrast, bariatric surgery has been shown to be the most effective means of weight loss and long-term weight loss maintenance in patients with obesity.1,5–7 In 1991, a consensus was reached by the National Institute of Health (NIH) stating that surgical treatment of obesity offers the best long-term results.3 A meta-analysis revealed that Roux-en-Y gastric bypass (RYGB) resulted in an average percent excess weight loss of 56.7 to 66.5 percent 24 months after surgery.2 In addition, several investigators have reported long-term weight loss extending up to 10 years after the index operation.8
With an estimated 120,000-plus bariatric surgeries performed in the United States (US) in 2008, and more than 340,000 operations performed globally in 2011, the worldwide number of performed bariatric surgeries is increasing continuously.1,2,9,10 Today, the laparoscopic RYGB and laparoscopic sleeve gastrectomy (LSG) are the two bariatric procedures performed most frequently,6,9 whereas the RYGB had remained the most heavily studied and one of the most common operations performed worldwide for patients with morbid obesity who have failed medical management. In recent years, the LSG has gained popularity as a treatment method for obesity in the US.1,3,7,11 A recent paper looking at Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP) data from 2015 determined that out of the 135,413 patients, 67 percent underwent LSG, 29 percent RYGB, three percent adjustable gastric banding, and one percent duodenal switch, which is reflective of current procedural trends in the US.12,13
While the benefit of weight loss after bariatric surgery is well documented, extensive literature also exists on the secondary benefits of these procedures. A meta-analysis revealed that RYGB resulted in resolution or improvement of diabetes in 86 percent, hypertension in 68 percent, obstructive sleep apnea in 81 percent, and hyperlipidemia in 97 percent of cases.2 Furthermore, bariatric surgery has also been found to have survival benefits, with some studies noting a 29-percent reduction in the long-term risk of death compared to conservative management.2
Despite the many benefits of bariatric surgery; however, it is undeniable that every intervention has the potential for adverse effects. Although bariatric surgery is considered safe, especially with the advent of laparoscopic techniques, complications, such as postoperative bleeding, still occur.10
Epidemiology and Risk Factors
Alongside with the usual postoperative and metabolic complications, luminal complications, such as anastomotic bleeding, ulceration, leakage, fistula formation, and enlargement or stenosis/obstruction of the anastomosis or gastric lumen can occur following bariatric surgery.1,9,10 In a study conducted by Daigle et al12 analyzing MBSAQIP data from 2015, it was determined that one of the most common complications after bariatric surgery was bleeding, accounting for 0.7 percent of reported complications in a sample of 135,413 patients. Other studies have reported the incidence of bleeding after bariatric surgery to be as high as 4.4 percent, especially in the early postoperative period (within the first hours to days) postsurgery in patients with obesity.3,5,7–10,14–24 Late bleeding (>30 days postoperative), however, is exceedingly rare, and although the actual incidence is unknown, there have been several case reports detailing its presentation.4 Rabl et al25 reported a series of seven patients out of 724 patients who presented with late hemorrhage. Heneghan et al26 noted 42 patients who developed postoperative bleeding out of 4,466 cases over a period of 10 years, 12 of whom presented with late bleeding (mean presentation of 553 days postoperative).
Several studies have aimed to examine risk factors associated with postoperative bleeding after bariatric surgery. Currently, there is contrasting literature regarding the use of low molecular weight heparin (LMWH) and the risk of postoperative bleeding in bariatric surgery patients. While some studies show no correlation between the use of LMWH and the risk of postoperative bleeding, other studies have demonstrated an increased incidence of early hemorrhage after RYGB in patients who received preoperative LMWH.10,21,23 In terms of antiplatelet agents, one study examined the effect of clopidogrel on postoperative bleeding in patients who had undergone gastric bypass and found a high incidence of bleeding (36%). Although their sample size was small, the high rate of bleeding suggests that even the rare patient taking clopidogrel has a higher risk of bleeding in the months after gastric bypass surgery compared to those who don’t take it.21,27 This could be due to the vulnerability of the anastomoses, even weeks or months after the operation. A different study examined ketorolac, an injectable anti-inflammatory drug with strong antiplatelet activity often used as an alternative to opioid analgesia, and determined that there was no correlation with incidence of perioperative bleeding in postbariatric surgery patients.10 It is also hypothesized that the use of nonsteroidal anti-inflammatory drugs (NSAIDs), helicobacter pylori infection, and smoking can increase the risk of gastrointestinal (GI) bleeding after bariatric surgery due to the role these factors play in the pathogenesis of ulcer formation at the anastomoses.9,28 Lastly, several studies have demonstrated a higher incidence of early postoperative bleeding in bariatric surgery patients undergoing laparoscopic surgery compared to those undergoing open gastric bypass.7,10,14,18,19,21,23,29
Although the literature demonstrates the incidence of bleeding after bariatric surgery to be uncommon, one study examining the top causes for readmission after LSG determined that complications related to the surgery (e.g., bleeding, leak) were the second-most common cause for readmission within one year of the index operation.1 A different study examining readmission rates within 30 days after bariatric surgery had similar findings, with bleeding accounting for 5.8 percent of surgery-related readmissions.13
Bleeding after bariatric surgery can prove to be catastrophic. In fact, bleeding and leak have been shown to have the largest overall effect on end-organ dysfunction, reoperation, and intensive care unit (ICU) admission after bariatric surgery.12 A study examining outcomes of early postoperative bleeding after gastric bypass surgery found that 15 percent of bleeding patients required ICU admission, and 31 percent required reoperation, and, compared to nonbleeding patients, those with early postoperative bleeding had a significantly longer hospital stay and a greater mortality rate.14,20,21,23 This underscores the importance that clinicians understand the differential diagnoses to consider when encountering a case of potential bleeding after bariatric surgery.
Differential Diagnoses
Like most postoperative patients who present with bleeding, bariatric surgery patients who present with acute hemorrhage will typically exhibit tachycardia, hypotension, and decreased urine output.3 One study noted that in patients with postoperative bleeding after bariatric surgery, the most common presenting symptom was tachycardia (46%) followed by melena (32%) and hematemesis (18%).14 However, the presentation of postoperative bleeding in this subset of patients can vary based on the timing (early onset vs. late onset) and location (intraluminal vs. extraluminal). The definition of early onset bleeding varies throughout the literature, but for the purposes of this review, early onset is classified as bleeding occurring less than 72 hours postoperatively.
Although bleeding overall is uncommon in the early postoperative period after bariatric surgery, the most common causes are usually due to bleeding from the anastomosis or staple line.4,17–19,27 Depending on the procedure, these can include the sleeve staple line, if the patient underwent a LSG, or the gastrojejunostomy, gastric pouch, jejuno-jejunostomy, or the bypassed remnant stomach, if the patient underwent a RYGB.2,7,14,16,18,19,21,23,29 In a study by Escalante-Tattersfield et al17 the most commonly identified sites of bleeding following bariatric surgery were the gastrojejunostomy (21%), or the jejuno-jejunostomy (21%) staple lines, and within the gastric remnant (14%).
Again, it is important to differentiate whether bleeding is occurring from an intraluminal or extraluminal source. Bright red blood per mouth (hematemesis), bright red blood per rectum (hematochezia), and melena are all indicators of an intraluminal process, as depicted in Figure 1.2–5,9,10,14,16,18,20,21,23,24,27 However, it must be noted that the presence of melena without other clinical manifestations, such as change in hematocrit or vital signs, usually represents the evacuation of old blood clots that accumulated from the primary procedure and usually does not represent active bleeding.18
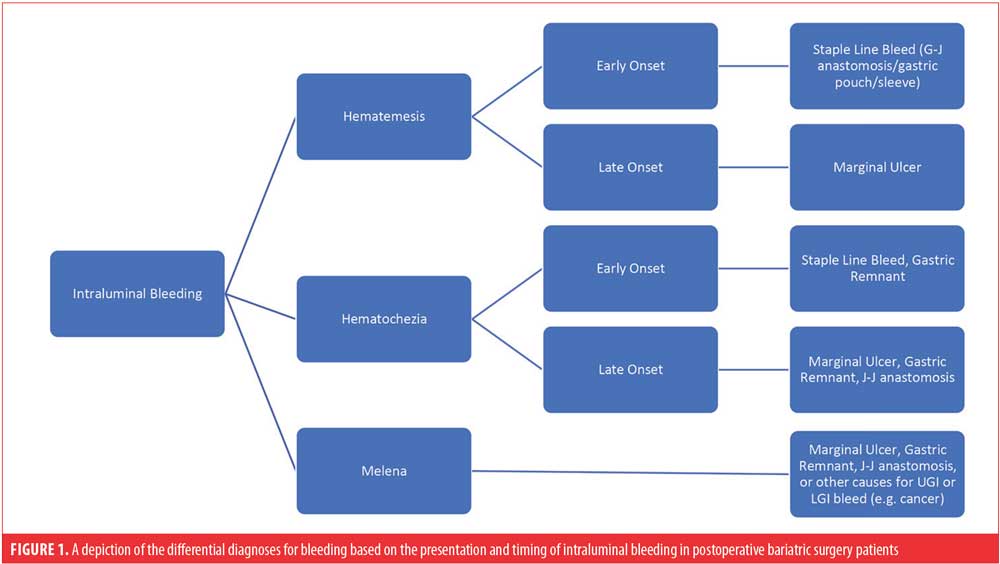
Marginal ulcerations are mostly found directly at the anastomosis itself or near the gastrojejunostomy a few weeks or months postsurgery.2,6,9 They are consistently reported in the literature as complications of both open and laparoscopic RYGB, with an incidence ranging from 0.6 to 20 percent.2,4,6,9,11 Etiology of these ulcers is multifactorial: contributing factors can be patient-related (e.g., presence of diabetes with microvascular disease, NSAID therapy, or tobacco use) or procedure-related factors (e.g., anastomotic tension, increased gastric acid production, helicobacter pylori infection, or foreign material such as sutures).2,4,6,9,11,30
In contrast, patients who present with bleeding from an extraluminal source following bariatric surgery typically do not exhibit the cardinal symptoms of GI bleeding (i.e., melena, hematochezia, and hematemesis).10 Rather, these patients typically present similarly to most patients with postoperative bleeding, with symptoms of persistent tachycardia, hypotension, low urine output, and possibly abdominal pain with distension.2,10 If a drain is left in the abdominal cavity, one can expect to see increased drain output unless it has clotted, in which case the drain will not assist in making the diagnosis.3,10,17,24 Causes for extraluminal bleeding in the early postoperative period can include staple lines (as the most common), abdominal wall trocar sites, and mesentery of the small bowel.3 Regardless of the presentation, the identification of the site of bleeding is crucial to determine the subsequent management of these patients.16
Workup/Management/Outcomes
First, like any case of postoperative bleeding, in bariatric surgery patients, one must stabilize the patient with adequate fluid resuscitation in preparation for blood transfusions.18,21 Additionally, the clinician should obtain a full set of labs and coagulation studies as well as withdraw any antithrombotic therapies.10,21 In most cases, the bleeding will be self-limited, resolving after discontinuation of anticoagulation.10,14 Occasionally, however, bleeding can result in significant postoperative morbidity and mortality.
There is ongoing debate about the role of imaging in the investigation of bleeding in bariatric patients postsurgery. Some investigators suggest that diagnostic studies are unnecessary because the etiology of bleeding is likely to be at one of the staple lines.10,18,23,29 However, other reports encourage an abdominal imaging study (e.g., computed tomography [CT] scan) in the presence of alarming symptoms, such as tachycardia, oliguria, abdominal distention, and changes in hematocrit.10 The clinician should use his or her best judgment, taking into consideration the patient’s presentation and condition, when deciding whether to obtain abdominal imaging.
Next, as previously described, the workup and treatment will depend on the clinical presentation and timing. If there is concern for early postoperative extraluminal bleeding with signs of hemodynamic instability, such as persistent hypotension, tachycardia, and low urine output, then the patient should be taken to the operating room immediately for diagnostic laparoscopy with possible conversion to laparotomy.17,18 The primary goal during re-exploration should be to evacuate the majority of the blood clots and oversew all staple lines. It is not necessary to identify the actual site of bleeding because it is often too difficult and not accomplished in most cases.14,18,29 However, if the patient is hemodynamically stable, the decision could be made to proceed with observation and fluid, with blood replacement as a conservative management option.7,10,14,17,22,24 There are various reports in the literature noting that around 75 percent of cases of postbariatric surgery bleeding resolve without the need for further diagnostic or therapeutic interventions.3,7,10 If there is suspicion for early postoperative intraluminal bleeding and the patient is hemodynamically stable, conservative management can be pursued, as previously described.7,14,17,22,24 However, if the patient exhibits symptoms of continued bleeding, the workup can be variable because there are more diagnostic and therapeutic options to consider. The role of early endoscopy in postbariatric surgery patients has been widely debated in the literature.22 There has been hesitation to perform an esophagogastroduodenoscopy (EGD) in the immediate postoperative period due to concern for disruption of the anastomosis with subsequent dehiscence or perforation.2,7,20–22,24,29 However, recent reports have deemed endoscopy safe to perform in the early postoperative period following bariatric surgery.7,20,22,23 Some investigators even regard endoscopy as the most accurate and practical method for diagnosing and treating the source of upper GI bleeding in this patient population.7,9,22,23 Of note, if early endoscopy is not available, cross-sectional imaging should be considered because it can aid in the diagnostic workup.9
Endoscopic intervention can be effective for making the diagnosis and treatment if the source of bleeding is the gastric pouch or the gastrojejunostomy, in which case endoscopy should be the first line of therapy.14,16,18,28,29 If possible, EGD should be performed in the operating room under general anesthesia with endotracheal intubation to provide for controlled conditions and minimize the risk of aspiration in this high-risk group of patients.20 Ideally, the bariatric surgeon should be present in the event that subsequent operative intervention is necessary.10,14,20 Additionally, carbon dioxide is preferred to air insufflation, and the endoscopist should understand the importance of using minimal insufflation or clamping the bowel (if concomitant laparoscopy is performed).2,9,10,22
Evolution of interventional endoscopy frequently allows endoscopic management of postoperative bleeding, avoiding surgical interventions in most cases.9,21 Devices used for endoscopic hemostasis in the GI tract can be classified into four categories: injection devices, mechanical devices, thermal devices, and topical devices (Table 1).9,10,16,18,21,22,31,32 It is worth emphasizing that thermal techniques should be used with caution along the staple line in the immediate postoperative period. Favor should be given to mechanical methods of hemostasis, such as endoclips, whenever technically feasible.2,22 Unlike sclerosant injection and thermal coagulation, endoscopic clips do not produce additional tissue injury and can be used to manage concurrent anastomotic leaks and iatrogenic perforations.10,21 In a subgroup of patients from a large meta-analysis, no combination of treatments was superior to mechanical therapy with hemostatic clips in patients with high-risk bleeding ulcers.31
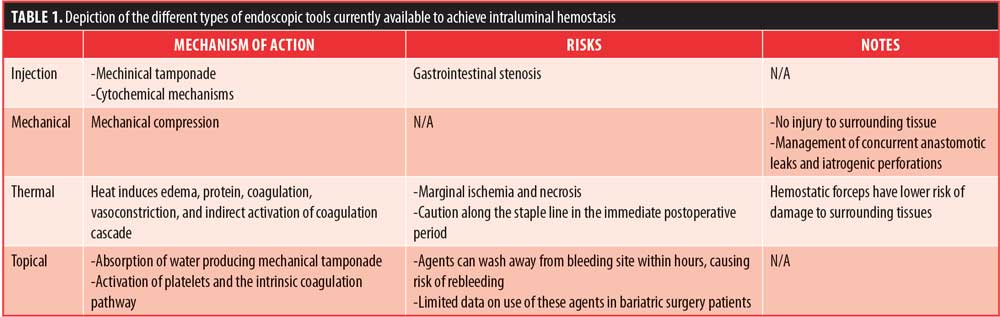
Overall, the use of these standard hemostatic modalities have been reported in several small series as effective and safe management techniques for intraluminal bleeding.4,5,20,22 Endoscopic treatment of upper GI hemorrhage has been 100-percent effective in some of these studies, with less than 17 percent requiring a second endoscopic intervention in most of the studies.10,14,21 In their series, Fernandez-Esparrach et al7 achieved hemostasis endoscopically in all cases of early intraluminal postoperative bleeding, with no damage to the anastomosis.7 Nonetheless, a contrast study might be beneficial after any endoscopic therapy to rule out any potential perforations.18 If endoscopic treatment is unsuccessful, prompt surgical exploration is indicated to control the bleeding.14
While the esophagus, pouch, and gastrojejunal anastomosis are easily accessible with a standard gastroscope, bleeding in the excluded stomach or at the jejunojejunal anastomosis presents a more difficult challenge.2,4,14,18 Of note, with bleeding in the gastric remnant, obtaining an abdominal CT scan will usually show a dilated remnant with blood clots.4 Until recently, patients would undergo intraoperative endoscopy through a gastrotomy in an effort to find the etiology of bleeding and evacuate the clot to relieve the obstruction.14,15,18 Sundblom and Silecchia24 also promoted CT and/or ultrasound guided percutaneous access to the bypassed stomach for EGD or virtual endoscopy. However, both methods are time-consuming and unsuitable for the evaluation of immediate postoperative hemorrhage.24 Overtube-assisted enteroscopy (single- or double-balloon enteroscopy) is usually needed to assess the distal anastomosis.21 This technique enables the endoscopist to reach the major duodenal papilla and the bypassed portion of the stomach in the setting of RYGB anatomy.9 A study in 2005 demonstrated the effectiveness of double-balloon enteroscopy in interrogating the gastric remnant. The bypassed stomach was reached in 5 of 6 (83%) patients. This method was not associated with the morbidity or risks of the additional surgical interventions required to visualize the gastric remnant.15 However, push enteroscopy or device-assisted enteroscopy poses its own risks due to the forces applied to the small bowel during the procedure.2,10,21 Therefore, although the use of the double balloon technique made it possible to observe the gastric remnant and duodenum after laparoscopic RYGB, the sample size was small, and a study with greater power is needed to confirm the safety of this procedure.14
In the case that endoscopic procedures are not successful in identifying the source of bleeding, there have been reports of successful angiography with subsequent embolization to provide hemostasis.10,16,28 An important complication of embolization is the risk of ischemia to the organ supplied by the target blood vessel.2,10 This is particularly concerning when considering that the small gastric pouch after RYGB is mainly supplied by the left gastric artery, which, if embolized, could lead to pouch ischemia and necrosis.16 Operative intervention might be necessary if angiography fails or is not readily available.28
Late hemorrhage most often arises secondary to marginal ulcers, and these patients should undergo EGD to visualize the gastric pouch, gastrojejunal anastomosis, and the proximal Roux limb.2 In the presence of a foreign body, such as sutures, they should be extracted when possible.2 Otherwise, in hemodynamically stable patients, the management generally requires acid suppression with medical therapy, smoking cessation, discontinuation of NSAIDs, optimization of diabetes, and/or treatment of Helicobacter pylori infection.2,4–6
Prevention
Prevention of postoperative bleeding in bariatric surgery patients, includes several modifications to the operative technique. One improvement is the use of staple line reinforcement devices.7,14,18,19,29 Both permanent and nonpermanent staple line buttressing materials have been used in gastric bypass and vertical sleeve gastrectomy surgeries.14,18 Several small studies have demonstrated a reduction in intraoperative and postoperative staple line bleeding with the use of staple line reinforcement materials.14,19,24 A study by Shikora et al14 demonstrated no episodes of GI hemorrhage with the use of bovine pericardial strips in 250 consecutive patients. Similarly, De la Torre et al33 reported the use of Seamguard® (W.L. Gore & Associates, Newark, Delaware) on 50 laparoscopic RYGB patients resulted in no occurrences of postoperative GI hemorrhage. It is important to note that the risk of staple-line reinforcement sleeves is related to the possibility of staple misfire and staple malformation due to the added thickness of the biomaterial.19
Another alteration to the bariatric surgery operative technique is the meticulous inspection of the jejunojejunostomy staple line.14 This technique is performed by inverting the staple line and using cautious electrocautery to halt any bleeding seen during surgery. Since adopting the technique, Dick et al14 reported no episodes of bleeding from the jejunojejunostomy site. The technique of inspecting the staple line has also been used to inspect the posterior staple line of the gastrojejunostomy.14
A linear stapler with a shorter staple height has also been used to prevent staple line bleeds.7,18,19,21,29 The shorter staple height provides more compression of the tissues and hence results in better hemostasis.18,19 One study reported that since downsizing staples on the stomach and the small bowel, they have had only one postoperative bleed requiring transfusion in the last 100 patients.8 Some investigators also suggested maintaining pressure after firing of the circular stapler for 30 seconds before release.7
Other prevention methods include reinforcement of the anastomosis and/or use of biological glue.21 Reinforcement of the anastomosis with oversewing of all staple lines during the primary operation has been proposed in the literature; however, this is a time-intensive task that increased times.18,29
The most common cause of late-onset bleeding after bariatric surgery is ulceration. Therefore, in an effort to prevent late-onset bleeding in these patients, efforts should be made to minimize the risk factors for the development of ulcers. From a procedural standpoint, reducing the size of the gastric pouch has been shown to decrease the incidence of marginal ulcers.6 Sapala et al34 found that creating a pouch limited to the cardia resulted in a 0.01-percent marginal ulceration rate at one year among 173 patients who underwent bariatric surgery. Finally, patients should also be counseled regarding the cessation of tobacco products and NSAIDs because these two factors have been consistently linked to higher rates of marginal ulcers.28
Conclusion
In summary, bariatric surgery offers an effective form of weight loss with minimal serious complication rates. Although overall rare, one of the more significant complication can be postoperative bleeding.12 Given the altered anatomy in these patients, it is important to understand the approach when developing a differential diagnosis, the workup, and the management of this potential life-threatening complication. The most common cause of postoperative bleeding is usually due to bleeding from the anastomosis or staple line.4,17–19,26 Although there has previously been hesitation to perform an EGD in the immediate postoperative period due to concern for disruption of the anastomosis or staple line, more recent reports have deemed endoscopy safe to perform in the early postoperative period after.2,7,20–24,28 With recent advancements in endoscopy, the clinician now has a wider scope of options for both diagnosing and treating postoperative intraluminal bleeding. In general, endoscopic tools for achieving hemostasis are categorized into injection, mechanical, thermal, and topical materials or therapies—each with different risks, benefits, and levels of efficacy. Overall, the use of these endoscopic hemostatic modalities has been reported to effectively and safely manage intraluminal bleeding; however, if unsuccessful then prompt surgical exploration is indicated.4,5,14,20,22
References
- Altieri M, Yang J, Groves D, et al. Sleeve gastrectomy: the first 3 years: evaluation of emergency department visits, readmissions, and reoperations for 14,080 patients in New York State. Surg Endosc. 2018;32:1209–1214.
- Kumar N, Thompson C. Endoscopic management of complications after gastrointestinal weight loss surgery. Clin Gastroenterol Hepatol. 2013;11:343–353.
- Dillemans B, Sakran N, Van Cauwenberge S, et al. Standardization of the fully stapled laparoscopic Roux-en-Y gastric bypass for obesity reduces early immediate postoperative morbidity and mortality: a single center study on 2606 patients. Obes Surg. 2009;19:1355–1364.
- Gupta A, Shah MM, Kalaskar SN, et al. Late postopeartive bleeding after Roux-en-Y gastric bypass: management and review of the literature. BMJ Case Rep. 2018;11:e226271.
- De Palma GD, Forestieri P. Role of endoscopy in the bariatric surgery of patients. World J Gastroenterol. 2014;20(24):7777–7784.
- Rasmussen JJ, Fuller M, Ali MR. Marginal ulceration after laparoscopic gastric bypass: an analysis of predisposing factors in 260 patients. Surg Endosc. 2007;21:1090–1094.
- Fernandez-Esparrach G, Bordas J, Pellise M, et al. Endoscopic management of early GI hemorrhage after laparoscopic gastric bypass. Gastrointest Endosc. 2008;67(3):552–555.
- Schauer P, Ikramuddin S, Gourash W, et al. Outcomes after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Ann Surg. 2000;232(4):515–529.
- Valli PV, Gubler C. Review article including treatment algorithm: endoscopic treatment of luminal complications after bariatric surgery. Clinical Obesity. 2017;7:115–122.
- Ferreira L, Wong Kee Song L, Baron T. Management of acute postoperative hemorrhage in the bariatric patient. Gastrointest Endoscopy Clin N Am. 2011;21:287–294.
- El-Hayek K, Timratana P, Shimizu H, et al. Marginal ulcer after Roux-en-Y gastric bypass: what have we really learned?. Surg Endosc. 2012;26:2789–2796.
- Daigle C, Brethauer S, Tu C, et al. Which postoperative complications matter most after bariatric surgery? Prioritizing quality improvement efforts to improve national outcomes. Surg Obes Relat Dis. 2018;14:652–657.
- Berger E, Huffman K, Fraker T, et al. Prevalence and risk factors for bariatric surgery readmissions findings from 130,007 admissions in the metabolic and bariatric surgery accreditation and quality improvement program. Ann Surg. 2018;267:122–131.
- Dick A, Byrne TK, Baker M, et al. Gastrointestinal bleeding after gastric bypass surgery: nuisance or catastrophe?. Surg Obes Relat Dis. 2010;6:643–647.
- Puri V, Alagappan A, Rubin M, et al. Management of bleeding from gastric remnant after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2012;8:e3–e5.
- Simillis C, Fachiri M, Bonanomi G. A challenging gastrointestinal hemorrhage after gastric bypass treated with interventional radiology. Surg Obes Relat Dis. 2016;12:e59–e62.
- Escalante-Tattersfield T, Tucker O, Fajnwaks P, et al. Surgical management of postoperative bleeding after bariatric surgery. Surg Obes Relat Dis. 2007;3:326.
- Nguyen N, Longoria M, Chalifoux S, et al. Gastrointestinal hemorrhage after laparoscopic gastric bypass. Obes Surg. 2004;14:1308–1312.
- Nguyen N, Longoria M, Welbourne S, et al. Glycolide copolymer staple-line reinforcement reduces staple site bleeding during laparoscopic gastric bypass. Arch Surg. 2005;140:773–778.
- Jamil L, Krause K, Chengelis D, et al. Endoscopic management of early upper gastrointestinal hemorrhage following laparoscopic Roux-en-Y gastric bypass. Am J Gastroenterol. 2008;103:86–91.
- Garcia-Garcia M, Martin-Lorenzo J, Torralba-Martinez J, et al. Emergency endoscopy for gastrointestinal bleeding after bariatric surgery. Cir Esp. 2015;93(2):97–104.
- Campos J, Moon R, Teixeira A, et al. Endoscopic management of massive hemorrhage 12 h post laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2015;25:1981–1983.
- Bakhos C, Alkhoury F, Kyriakides T, et al. Early postoperative hemorrhage after open and laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2009;19:153–157.
- Mehran A, Szomstein S, Zundel N, et al. Management of acute bleeding after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2003;13:842–847.
- Rabl C, Peeva S, Prado K, et al. Early and late abdominal bleeding after Roux-en-Y gastric bypass: sources and tailored therapeutic strategies. Obes Surg. 2011;21:413–420.
- Heneghan HM, Meron-eldar S, Yenumula P, et al. Incidence and management of bleeding complications after gastric bypass surgery in the morbidly obese. Surg Obes Relat Dis. 2012;8:729–735.
- Caruana J, McCabe M, Smith A, et al. Risk of massive upper gastrointestinal bleeding in gastric bypass patients taking clopidogrel. Surg Obes Relat Dis. 2007;3:443–445.
- Lane B, Biedenbach A, Bolduc A, et al. Catastrophic UGI bleed in gastric bypass patients from ulcer erosion into the splenic artery: details of rapid surgical treatment. Surg Obes Relat Dis. 2015;11:S114.
- Nguyen N, Rivers R, Wolfe B. Early gastrointestinal hemorrhage after laparoscopic gastric bypass. Obes Surg. 2013;13:62–65.
- Sverden E, Mattsson F, Sonden A, et al. Risk factors for marginal ulcer after gastric bypass surgery for obesity. Ann Surg. 2016;263:733–737.
- Parsi M, Schulman A, Aslanian H, et al. Devices for endoscopic hemostasis of nonvariceal GI bleeding. VideoGIE. 2019;4:285–299.
- Barkun A, Moosavi S, Martel M. Topical hemostatic agents: a systematic review with particular emphasis on endoscopic application in GI bleeding. Gastrointest Endosc. 2013;77;692–700.
- De la Torre RA, Scott JS. Laparoscopic Roux-en-Y gastric bypass: a totally intra-abdominal approach—technique and preliminary report. Obes Surg. 1999;9:492–498.
- Sapala JA, Wood MH, Sapala MA, et al. Marginal ulcer after gastric bypass: a prospective 3-year study of 173 patients. Obes Surg. 1998;8:505–516.
Category: Past Articles, Review



