Resolution of Obstruction in a Patient with Hypertrophic Obstructive Cardiomyopathy Following Bariatric Surgery

by Paul Travers, MD; Mohamad Yamani, MD; and Victoria Gomez, MD
Dr. Travers is with the Department of Internal Medicine, Mayo Clinic in Jacksonville, Florida. Dr. Yamani is with the Department of Cardiovascular Disease, Mayo Clinic in Jacksonville, Florida. Dr. Gomez is with the Department of Gastroenterology and Hepatology, Mayo Clinic in Jacksonville, Florida.
Funding: No funding was provided for this article.
Disclosures: The authors have no conflicts of interest relevant to this article.
Correspondence: Paul Travers, MD; Email: [email protected]
Bariatric Times. 2023;20(7–8). Published online August 10, 2023.
Abstract
Hypertrophic cardiomyopathy (HCM) is a cardiac disease with known genetic predisposition and variable clinical presentation and illness severity, as well as associations with other conditions, such as obesity. Presence of a significant left ventricular outflow tract (LVOT) obstruction causes hypertrophic obstructive cardiomyopathy (HOCM), which is associated with worse clinical presentation at the time of diagnosis, as well as poorer prognosis. We present a patient who underwent bariatric surgery for weight loss and experienced resolution of LVOT obstruction, with concurrent symptom resolution.
Keywords: Hypertrophic cardiomyopathy, left ventricular outflow tract obstruction, obesity, bariatrics
Hypertrophic cardiomyopathy (HCM) is a cardiac disease with well-known genetic predisposition and widely varying clinical presentation and disease progression.1 A diagnosis can be made in patients with a left ventricular wall thickness greater than or equal to 15mm that cannot be otherwise explained by abnormal intracardiac pressures or myocardial infiltrative disease.2 Classically, this manifests as an asymmetric interventricular septal hypertrophy, defined as a septum to left ventricular free wall thickness ratio of greater than or equal to 1.3, although almost any myocardial segment can be involved.2 While patients can present asymptomatically, symptoms are often due to dynamic left ventricular outflow tract (LVOT) obstruction, which commonly accompanies HCM. This obstruction occurs due to contact between the mitral valve and the interventricular septum during systole. In these instances, the disease is referred to as hypertrophic obstructive cardiomyopathy (HOCM), and symptoms mirror that of many cardiac diseases, with the triad of exertional dyspnea, angina, and peripheral edema.3 Obstruction is typically evaluated using echocardiographic estimation of peak resting and dynamic LVOT gradients, defined as the difference between aortic and left ventricular pressures during rest and Valsalva maneuver, respectively, but can also be measured directly during left cardiac catheterization.4,5 Resting obstruction is defined as a peak resting gradient of 30mmHg or greater and carries prognostic significance as a predictor of sudden cardiac death (SCD).4 A majority of patients with HOCM do not have LVOT obstruction at rest, but a dynamic obstruction can be provoked with maneuvers that reduce left ventricular end-diastolic volume or increase left ventricular contractility, such as Valsalva maneuver or intraoperative premature ventricular contraction (PVC) induction. About 50 percent of patients with HOCM also have echocardiographic findings of systolic anterior motion (SAM) of the mitral valve, although this is not needed for diagnosis and can be present in many other cardiac conditions.4
While symptom severity is variable, factors such as obesity are increasingly being associated with increased left ventricular mass and progression of symptoms.6 We present the case of a patient with morbid obesity and HOCM with LVOT obstruction who underwent bariatric surgery with subsequent significant changes to the LVOT gradient during the postoperative course, correlating with clinical improvement. Informed patient consent was obtained for publication of this article.
Case Report
A 65-year-old male patient with morbid obesity (body mass index [BMI]: 40.3kg/m2) and HCM presented for a cardiac evaluation prior to bariatric surgery. Comorbidities included hypertension, hyperlipidemia, coronary artery disease, and myocardial infarction status poststenting in the right coronary artery. The patient was symptomatic with exertional dyspnea. Physical exam was notable for a Grade 1/6 systolic ejection murmur located at the left sternal border that increased to Grade 3/6 standing with Valsalva.
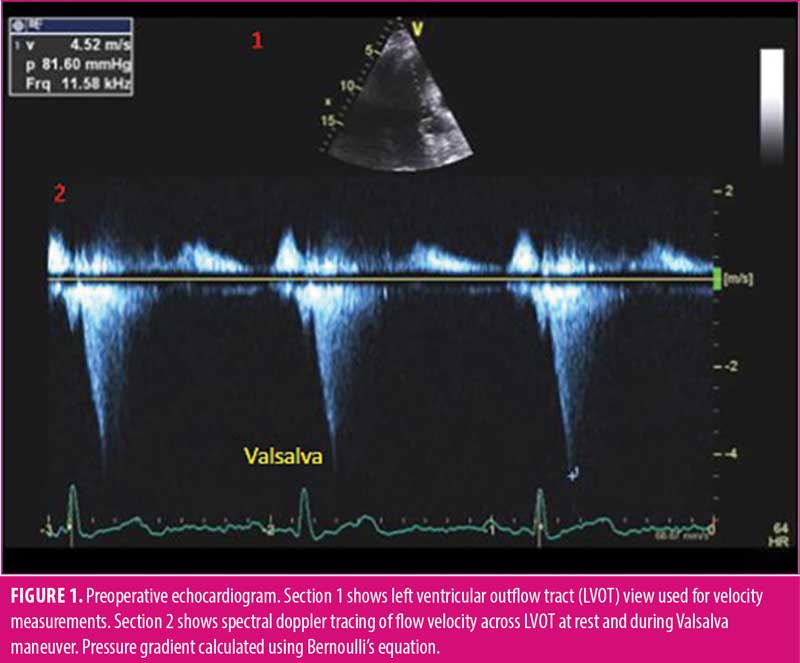
At presentation, standard echocardiogram demonstrated HOCM with asymmetric basal septal thickening of 17mm, ejection fraction of 74 percent, normal left ventricular chamber size, SAM of the anterior mitral leaflet with trivial mitral regurgitation, and a peak resting gradient of 12mmHg that increased to 81mmHg with Valsalva maneuver (Figure 1). Dobutamine stress echocardiogram revealed no pharmacologic-induced wall motion abnormalities, and target heart rate was successfully achieved. The patient was deemed to have a dynamic LVOT obstruction, compensated on medical therapy with preserved functional capacity to undergo intermediate risk procedure.
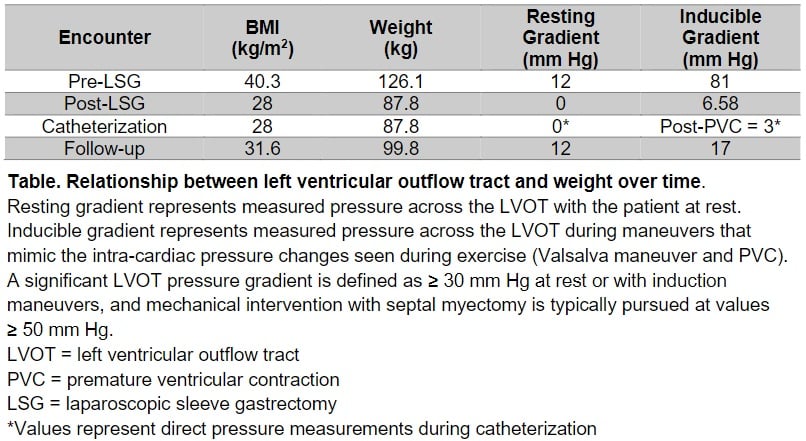
At the time of bariatric surgery, the patient weighed 127kg with a BMI of 39.8kg/m2. He underwent successful laparoscopic sleeve gastrectomy (LSG) with an uneventful postoperative course. Over the course of nine months, the patient lost a total of 39.2kg and reached a BMI of 28.0kg/m2 (Table 1).
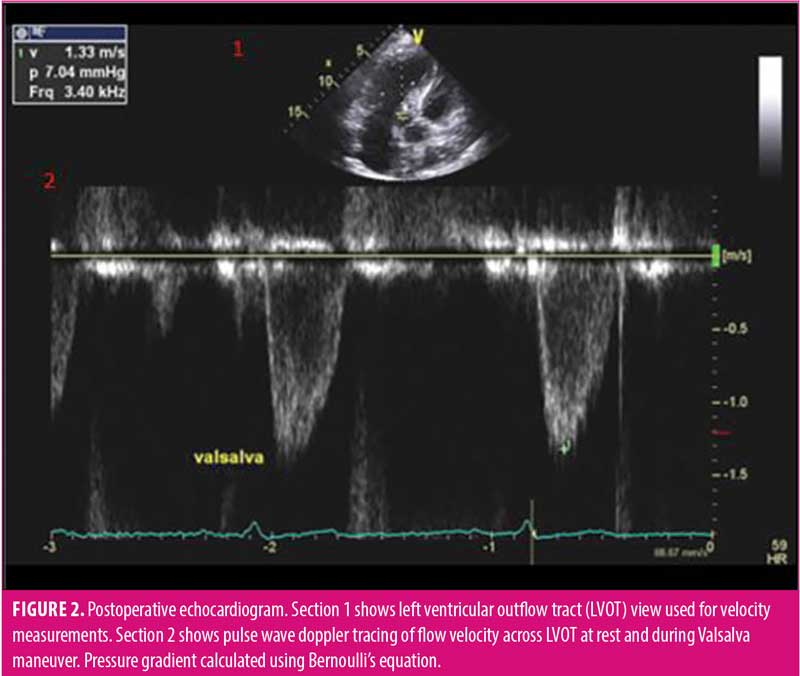
At his cardiology follow-up (9 months post-bariatric surgery), the patient reported improvement in dyspnea symptoms following weight loss. Repeat standard echocardiography at that time showed mildly increased concentric left ventricular wall thickness, but also reduction of peak resting gradient to 0mmHg and resolution of inducible gradient with Valsalva maneuver to 7.04mmHg (Figure 2). Ejection fraction was stable at 71 percent. Additionally, there was no longer any discrete SAM of the anterior mitral valve leaflet or mitral regurgitation. Blood pressure was uncontrolled during this follow-up visit (180/84) due to the patient reportedly running out of an antihypertensive prescription. A dobutamine stress test was obtained to rule out ischemic causes for this newly uncontrolled hypertension, which revealed dilatation of the left ventricle, consistent with ischemic changes. This led to a left heart catheterization, which revealed triple vessel disease. The patient underwent coronary artery bypass grafting (CABG) with a plan for possible septal myectomy. Intraoperative measurement of the left ventricular pressure and aortic pressure showed no gradient across the LVOT, and with induced PVCs, the gradient increased to 3mmHg. Therefore, it was concluded that there was no significant gradient across the LVOT, and septectomy was not performed.
Discussion
HCM is characterized by left ventricular hypertrophy (LVH) and is known to present with variable symptom severity.7 When symptoms do develop, they are due to a complex interplay of obstructed flow in the LVOT, inability of the coronary vessels to provide sufficient oxygenated blood to the hypertrophied myocardium, and diastolic dysfunction.3 In the subset of patients that develop obstruction, a number of pathophysiologic mechanisms have been identified, including the development of asymmetrical septal hypertrophy and systolic anterior motion of the mitral valve.3,8,9 Regardless of the cause, a diagnosis is made by assessing for the presence of a gradient across the LVOT using echocardiography or heart catheterization. Patients with HOCM will often not have a physiologically significant gradient at rest (defined as >30mmHg) but will develop one following provoking maneuvers, such as Valsalva and induced PVCs, that mimic the reductions to preload that occur during exercise.10 Once a diagnosis is made, management involves medical therapy with negative inotropes and/or mechanical intervention with septal myectomy or ablation.11
Obesity is a well-known risk factor for cardiovascular disease, diabetes mellitus, and chronic liver disease, including nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, cirrhosis, and hepatocellular carcinoma.12 Presence of obesity in patients with HCM is increasingly being associated with higher rates of obstruction and poorer long-term outcomes.13 In most cases, obesity is being studied as a concomitant condition that leads to increased complications, rather than a stimulating pathology. In 2017, a study published by Ingles et al14 examined a proposed nonfamilial subgroup of HCM and found that patients without a family history of the disease had a statistically significant increase in average BMI versus patients with a family history. Likewise, prevalence of obesity in the HCM population as a whole has been proven to be significantly higher than that of the general population.13 It is well established in the literature that LVH with maintained ejection fraction is a physiologic adaptation to the increased systemic oxygen requirements seen in obesity.15–17 However, in the landmark study published in 2013 by Olivotto et al6 examining the effects of obesity on patients with HCM, increases in BMI were powerfully associated with not only a severe left ventricular mass increase, but also a more than two-fold increase in prevalence of LVOT obstruction.6 While it is difficult to parse out the exact pathophysiologic mechanisms, it is clear that a significant relationship exists. In fact, there is evidence to suggest that obesity itself may predispose to the development of a nonfamilial variant of HOCM.
Conclusion
In conclusion, we present a case of a patient with symptomatic HOCM with a severe inducible LVOT gradient that resolved following significant weight loss achieved with bariatric surgery. Regardless of other cardiovascular risk factors, resolution of LVOT obstruction confers a prognostic improvement in patients with HOCM. While further research into their association is needed, this case demonstrates that obesity plays a role in the pathophysiologic development of obstruction in HCM, and early weight management interventions should be considered as part of the disease management in this patient population. National registries could consider including further objective data regarding cardiovascular risk factors in bariatric patients to further guide future clinical decision making.
Author Contributions
Paul Travers made substantial contributions to the conception or design of the work, as well as acquisition, analysis, and interpretation of the data; drafted the work and revised it critically for important intellectual content; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Mohamad Yamani and Victoria Gomez revised the work critically for important intellectual content and gave final approval of the version to be published.
References
- Wigle ED, Rakowski H, Kimball BP, Williams WG. Hypertrophic cardiomyopathy. Clinical spectrum and treatment. Circulation. 1995;92(7):1680–1692.
- Owens AT, Reza N. Diagnosis of hypertrophic cardiomyopathy: what every cardiologist needs to know. American College of Cardiology. 27 Feb 2020. https://www.acc.org/latest-in-cardiology/articles/2020/02/25/06/34/diagnosis-of-hypertrophic-cardiomyopathy. Accessed 26 Jan 2023.
- Fifer MA, Vlahakes GJ. Management of symptoms in hypertrophic cardiomyopathy. Circulation. 2008;117(3):429–439.
- Williams LK, Frenneaux MP, Steeds RP. Echocardiography in hypertrophic cardiomyopathy diagnosis, prognosis, and role management. Eur J Echocardiogr. 2009;10(8):iii9–iii14.
- Geske JB, Cullen MW, Sorajja P, et al. Assessment of left ventricular outflow gradient: hypertrophic cardiomyopathy versus aortic valvular stenosis. JACC Cardiovasc Interv. 2012;5(6):675–681.
- Olivotto I, Maron, BJ, Tomberli B, et al. Obesity and its association to phenotype and clinical course in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2013;62(5):449–457.
- Maron BJ. Hypertrophic cardiomyopathy: a Systematic review. JAMA. 2002;287(10):1308–1320.
- Teare D. Asymmetrical hypertrophy of the heart in young adults. Br Heart J. 1958;20(1):1–8.
- Nordenstrom B, Ovenfors CO. Low subvalvular aortic and pulmonic stenosis with hypertrophy and abnormal arrangement of the muscle bundles of the myocardium. ACTA Radiol. 1962;57(5):321–340.
- Maron MS, Olivotto I, Zenovich AG, et al. Hypertrophic cardiomyopathy Is predominantly a disease of left ventricular outflow tract obstruction. Circulation. 2006;114(21):2232–2239.
- Maron BJ, Mckenna WJ, Danielson GK, et al. American College of Cardiology/European Society of Cardiology Clinical expert consensus document on hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. J Am Coll Cardiol. 2003;42(9):1687–1713.
- Ritchie H, Roser M. Obesity. Our World in Data. 11 Aug 2017. https://ourworldindata.org/obesity. Accessed 26 Jan 2023.
- Fumagalli C, Maurizi N, Day SM, et al. Association of obesity with adverse long-term outcomes in hypertrophic cardiomyopathy. JAMA Cardiol. 2020;5(1):65–72.
- Ingles J, Burns C, Bagnall RD, et al. Nonfamilial hypertrophic cardiomyopathy: prevalence, natural history, and clinical implications. Circ Cardiovasc Genet. 2017;10(2):e001620.
- Canepa M, Sorensen LL, Pozios I, et al. Comparison of clinical presentation, left ventricular morphology, hemodynamics, and exercise tolerance in obese versus nonobese patients with hypertrophic cardiomyopathy. Am J Cardiol. 2013;112(8):1182–1189.
- Turkbey EB, McLelland RL, Kronmal RA, et al. The impact of obesity on the left ventricle: The Multi-Ethnic Study of Atherosclerosis (MESA). JACC Cardiovasc Imaging. 2010;3(3):266–274.
- Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiol Rev. 2008;88(2):389–419.
Category: Case Report, Online Only



