Hypoglycemia Post-bariatric Surgery: An Underdiagnosed Condition
by Lina Alkhaled, MD, and W. Scott Butsch, MD, MSc
Drs. Aklhaled and Butsch are with the Cleveland Clinic Foundation in Cleveland, Ohio.
Funding: No funding was provided for this article.
Disclosures: The authors have no conflicts of interest relevant to this article.
Bariatric Times. 2022;19(10):9–10.
Abstract
Postoperative hypoglycemia is an increasingly recognized complication of metabolic surgery that is both underreported and underdiagnosed. In this article, we present the case of 62-year-old female patient who was nine-years post-gastric bypass and had a recent diagnosis of hypoglycemia. We discuss the work-up that lead to the suspected diagnosis, in addition to highlighting symptomatology, pathophysiology, and differential diagnoses of post-bariatric surgery hypoglycemia.
Keywords: Metabolic surgery, hypoglycemia
Bariatric surgery is the most effective therapy for the treatment of severe obesity and related metabolic diseases. The long-term improvements in obesity-related complications and cardiovascular risk factors after bariatric surgery have been well established,1,2 including prediabetes and Type 2 diabetes mellitus.3
Despite both weight-dependent and weight-independent improvements in glycemic control after bariatric surgery, some patients develop postoperative hypoglycemia, a known complication that is both underdiagnosed and underreported. Different mechanisms have been proposed as underlying causes of hypoglycemia post-bariatric surgery; accurate identification of the cause, however, might not be feasible, given the overlap in the mechanisms and the lack (and sometimes the infeasibility) of gold standards to confirm the diagnosis.
In this article, we will review a case of a 62-year-old female patient who underwent gastric bypass nine years prior to presentation. The patient had a history of autoimmune thyroiditis and osteoporosis, for which she was followed by our endocrine team. During her follow-up visit, the patient reported symptoms of hypoglycemia that had been going on for several years, which she had attributed to possible dumping. However, a formal work-up and diagnosis were never done. Laboratory tests, including renal function panel, were done as part of routine work-up. Results showed a critically low glucose level at 33mg/dL; otherwise, her laboratory results were within normal limits. She reported having a well-balanced meal 2 to 3 hours before the labs were drawn, but denied having any hypoglycemia-related symptoms at the time of the blood draw.
The aim of this article is to highlight the importance of identifying hypoglycemia in patients with history of bariatric and metabolic surgery. The management and treatment of hypoglycemia will not be discussed in this article.
Postoperative Hypoglycemia
Understanding the mechanism of improved postoperative glycemic control can provide better insight into the causes of the exaggerated responses that lead to postoperative hypoglycemia. Proposed mechanisms of improved postoperative glycemic control include restriction of caloric intake, with subsequent improvement in insulin sensitivity. In addition, with the gastric bypass, the fast transit and emptying of bile and nutrients into the small intestine, bypassing the duodenum and proximal jejunum, and removal of the gastric fundus lead to improvement of glycemic control.4 On a hormonal level, changes in the gastrointestinal hormones that communicate with peripheral tissues and the central nervous system (CNS) play a significant role in regulating glucose homeostasis and energy balance. The gut hormones of interest are peptide YY (PYY) and glucagon-like peptide-1 (GLP-1), which are secreted by distal intestinal L-cells, in addition to ghrelin, which is secreted by the gastric fundus and proximal small intestine. While PYY and GLP-1 both decrease appetite, increase satiety, and slow gut motility, PYY also improves insulin sensitivity, while GLP-1 functions as an incretin to potentiate glucose-stimulated insulin release, in addition to having an antiapoptotic effect on B cells, which results in increased B cell mass.5
When evaluating for postoperative hypoglycemia, one of the first steps is to differentiate it from dumping syndrome, as symptoms (e.g., dizziness, palpitations, fatigue, shakiness, hunger) might overlap. Typically, postprandial blood glucose levels are within normal limits with dumping syndrome; however, mild hypoglycemia (i.e., blood glucose 50–70mg/dL) has occurred in a few cases.6 Nonetheless, confirming hypoglycemia is important. The next step is to identify the timing of hypoglycemia to surgery (early vs. late postoperative phase) and food intake (early, within 45 minutes, vs. late, within 1–3 hours). Table 1 summarizes different causes of postoperative hypoglycemia and dumping syndrome.
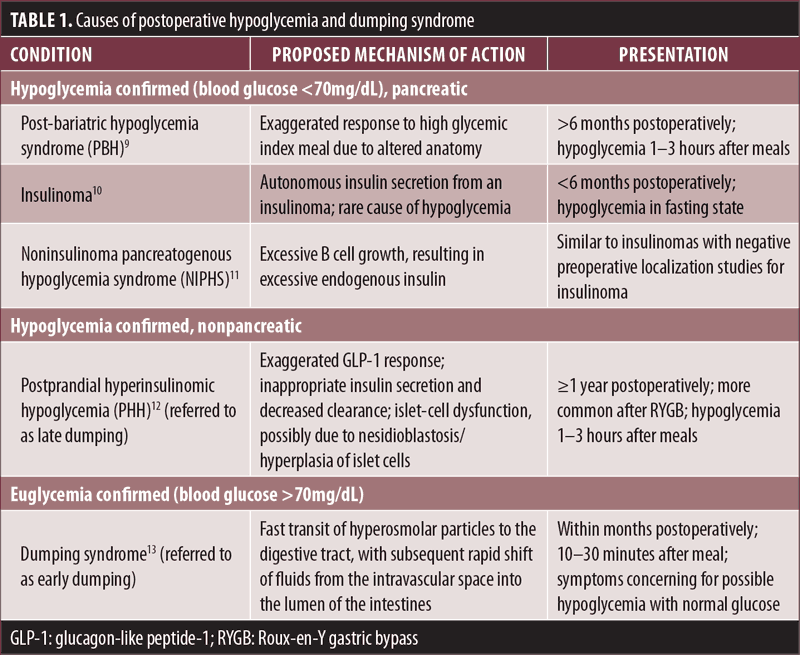
As outlined in our case, postoperative hypoglycemia is difficult to diagnose. Some patients might have mild or absent symptoms, while others might present with symptoms that could be confused with dumping syndrome. We believe that hypoglycemia post-bariatric surgery is potentially more common than reported. In a Swedish nationwide study, it was found that the prevalence of postoperative hypoglycemia in a cohort of 5,040 patients who underwent bariatric surgery was close to 0.2 percent.7 Brix et al,8 in a prospective study of a two-year observation period after bariatric surgery and using a two-hour oral glucose tolerance test (OGTT) to assess blood glucose and insulin levels, found that a high percentage of patients experienced hypoglycemia postoperatively. Hypoglycemia was the highest after gastric bypass (32.6%), followed by sleeve gastrectomy (22.6%), but only 2.3 percent of patients had hypoglycemia after gastric banding.8
Currently, there are no standardized guidelines for evaluation, diagnosis, or management of postoperative hypoglycemia. Thus, a detailed history screening for symptoms of hypoglycemia should take place at each postoperative clinical encounter. After ruling out exogenous causes of hypoglycemia (e.g., sulfonylurea intake), suggested screening modalities may include the 72-hour fasting plasma glucose test (to evaluate for fasting vs. postprandial hypoglycemia) and mixed meal tolerance test (to assess postprandial hypoglycemia and corresponding insulin levels). All testing can be done in outpatient settings. Once hypoglycemia is identified, appropriate referrals should be placed to a registered dietitian nutritionist (RDN) for medical nutrition therapy (MNT), as this remains the cornerstone of treatment to prevent or reduce the frequency and severity of hypoglycemia. The use of pharmacological agents should be considered as adjunctive therapy.
Case Report
The management of our patient included an initial 72-hour fasting plasma glucose test. However, she was only able to fast for 15 to 17 hours, and the test was ended earlier, with no evidence of hypoglycemia during the test. A mixed meal tolerance test demonstrated hypoglycemia, with a glucose level of 49mg/dL (reference range: 74–99mg/dL) and hyperinsulinemia of 185.5mU/L (reference range: 3.0–25.0 mU/L) at the three-hour mark. She was asymptomatic at that time. Continuous glucose monitoring (CGM) in blinded mode was then utilized, which confirmed frequent daily postprandial hypoglycemia with minimal or no associated symptoms. She was thought to have signs and symptoms of potential postprandial hyperinsulinomic hypoglycemia (PHH; also known as late dumping). Referral to a RDN for MNT was placed, and pharmacological therapy was considered if symptoms persisted.
Conclusion
Our understanding of the pathophysiology of hypoglycemia following bariatric surgery has advanced in the past decade. However, there are yet to be evidenced-based guidelines for the diagnosis and treatment of postoperative hypoglycemia. Early identification of patients with autonomic/neuroendocrine and neuroglycopenic symptoms is warranted to avoid hypoglycemia and its complications. Patients could benefit from multidisciplinary care, with a spectrum of interventions, ranging from early self-directed diet changes to a surgical revision of the primary bariatric surgery. Shared decision-making throughout the care of the postoperative bariatric surgery patient, especially in these clinical situations, is important.
References
- Aminian A, Zajichek A, Arterburn DE, et al. Association of metabolic surgery with major adverse cardiovascular outcomes in patients with type 2 diabetes and obesity. JAMA. 2019;322(13):1271–1282.
- Albaugh VL, Kindel TL, Nissen SE, et al. Cardiovascular risk reduction following metabolic and bariatric surgery. Surg Clin North Am. 2021;101(2):269–294.
- Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes–5-year outcomes. N Engl J Med. 2017;376(7):641–651.
- Batterham RL, Cummings DE. Mechanisms of diabetes improvement following bariatric/metabolic surgery. Diabetes Care. 2016;39(6):893–901.
- Goldfine AB, Mun EC, Devine E, et al. Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. J Clin Endocrinol Metab. 2007;92(12):4678–4685.
- Van Furth AM, de Heide LJM, Emous M, et al. Dumping syndrome and postbariatric hypoglycemia: supporting evidence for a common etiology. Surg Obes Relat Dis. 2021;17(11):1912–1918.
- Marsk R, Jonas E, Rasmussen F, et al. Nationwide cohort study of post-gastric bypass hypoglycaemia including 5,040 patients undergoing surgery for obesity in 1986-2006 in Sweden. Diabetologia. 2010;53(11):2307–2311.
- Brix JM, Kopp H-P, Höllerl F, Schernthaner GH, et al. Frequency of hypoglycaemia after different bariatric surgical procedures. Obes Facts. 2019;12(4):397–406.
- Sarwar H, Chapman WH, 3rd, Pender JR, et al. Hypoglycemia after Roux-en-Y gastric bypass: the BOLD experience. Obes Surg. 2014;24(7):1120–1124
- Mulla CM, Storino A, Yee EU, et al. Insulinoma after bariatric surgery: diagnostic dilemma and therapeutic approaches. Obes Surg. 2016;26(4):874–881.
- Anderson B, Nostedt J, Girgis S, et al. Insulinoma or non-insulinoma pancreatogenous hypoglycemia? A diagnostic dilemma. J Surg Case Rep. 2016;2016(11):rjw188.
- Salehi M, Gastaldelli A, D’Alessio DA. Altered islet function and insulin clearance cause hyperinsulinemia in gastric bypass patients with symptoms of postprandial hypoglycemia. J Clin Endocrinol Metab. 2014;99(6):2008–2017.
- Van Beek AP, Emous M, Laville M, Tack J. Dumping syndrome after esophageal, gastric or bariatric surgery: pathophysiology, diagnosis, and management. Obes Rev. 2017;18(1):68–85.
Category: Obesity Medicine Fellow Corner, Past Articles



