Quantitative Versus Qualitative Monitoring of Neuromuscular Blockade in Patients with Morbid Obesity Undergoing Bariatric Surgery: Differences in Residual Neuromuscular Block and Respiratory Complications
 by P. Ziemann-Gimmel, MD; Allison A. Goldfarb, DNP, CRNA; Meghan Connelly, MSN, CRNA; Ahmed Bata, MD; Robert Marema, MD; and Dmitry Tumin, PhD
by P. Ziemann-Gimmel, MD; Allison A. Goldfarb, DNP, CRNA; Meghan Connelly, MSN, CRNA; Ahmed Bata, MD; Robert Marema, MD; and Dmitry Tumin, PhD
Drs. Ziemann-Gimmel and Bata and Mses. Goldfarb and Connelly are from the Department of Anesthesiology, Envision Healthcare Inc, Plantation, Florida, United States. Dr. Marema is from the Department of Surgery, Flagler Hospital, St. Augustine, Florida, United States. Dr. Tumin is from the Center for Epidemiology of Organ Failure and Transplantation, Nationwide Children’s Hospital, Columbus, Ohio, United States.
Reprinted with permission from the authors. Ziemann-Gimmel et al © Copyright 2018.
J Surg: JSUR-1125. DOI: 10.29011/2575-9760. 001125
COPYRIGHT: © The Authors. 2018. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Funding: This project was supported by Flagler Hospital (equipment and respiratory therapist work hours), St. Augustine, Florida, and Envision Healthcare Inc. (CRNA work hours), Plantation, Florida. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosures: The author reports no conflicts of interest relevant to the content of this manuscript.
Abstract: Background: Patients with morbid obesity are at high risk of developing respiratory complications in the postoperative period following bariatric surgery. The literature suggests that quantitative management of neuromuscular blockade reduces the residual weakness that can lead to critical respiratory events in the postoperative period. Thus, we investigated which approach, qualitative or quantitative monitoring of neuromuscular blockade, left patients with a degree of residual neuromuscular block that created a greater risk of having critical respiratory events. Methods: Patients with morbid obesity undergoing bariatric operations were enrolled in a prospective, randomized parallel-group, single-center trial. In the control group, administration of the reversal agent, neostigmine, and tracheal extubation was based on qualitative monitoring of neuromuscular blockade, whereas in the intervention group it was based on quantitative measurements via acceleromyography. Results: By study design, there was no difference in the intraoperative dosing of intermediate neuromuscular blocking agents. The control group showed a mild degree of residual neuromuscular block relative to the intervention group at the time of extubation (train-of-four ratio: mean 0.86 vs 0.94). There was no difference in the occurrence of critical respiratory events in the postanesthesia care unit. After 317 of the 362 patients were enrolled, sugammadex was added as a reversal agent at the study facility. As a result, the study was terminated early. A futility analysis indicated that the study had less than one percent conditional power for rejecting the null hypothesis if the target enrollment had been achieved. Conclusion: Quantitative monitoring of neuromuscular blockade reduced the amount of residual neuromuscular blockade at the time of extubation. However, it did not decrease the risk of critical respiratory events.
Introduction
Background. Several studies have identified chronic obstructive pulmonary disease (COPD), long surgeries, old age, high American Society of Anesthesiologists (ASA) score, a history of congestive heart failure, emergency surgery, and functional dependence as main risk factors for triggering postoperative pulmonary complications.1,2 Qualitative relative to quantitative management of neuromuscular blockade predisposes patients to postoperative residual neuromuscular block.3 Postoperative residual neuromuscular block seems to be a risk factor for postoperative pulmonary complications and critical respiratory events.4 Moreover, patients with morbid obesity are at risk for postoperative respiratory events.5
Rationale/significance. Our current clinical practice of monitoring neuromuscular blockade uses a standard peripheral nerve stimulator to stimulate the facial nerve (qualitative) and visually assess the response of the corrugator supercilii muscle (CS). The CS is more resistant to the effect of neuromuscular blocking agents, requires higher concentrations/doses, and leaves patients with a considerably high degree of postoperative residual neuromuscular block.6,7 It is recommended to use quantitative monitoring at the adductor pollicis muscle (AP) to avoid postoperative residual neuromuscular block.8
Study objectives/aims. The aim of the study was to determine whether the risk of postoperative residual neuromuscular block and respiratory complications could be decreased by using quantitative neuromuscular monitoring (acceleromyography [AMG]) compared to our current clinical practice, using qualitative (visual) neuromuscular monitoring. We hypothesized that patients in the control group using qualitative (visual) assessment have a higher incidence of respiratory complications than patients in the study group using AMG of the AP.
Materials and Methods
Trial design. This prospective, randomized, parallel-group, single-center study was conducted at Flagler Hospital in St Augustine, Florida. The study complied with the Declaration of Helsinki and was approved by the local Institutional Review Board (Flagler Life Institute, St Augustine, Florida, #00006886). All participating patients gave written consent. The study was registered at clinicaltrials.gov NCT02037516 (https://clinicaltrials.gov/ct2/show/NCT02037516).
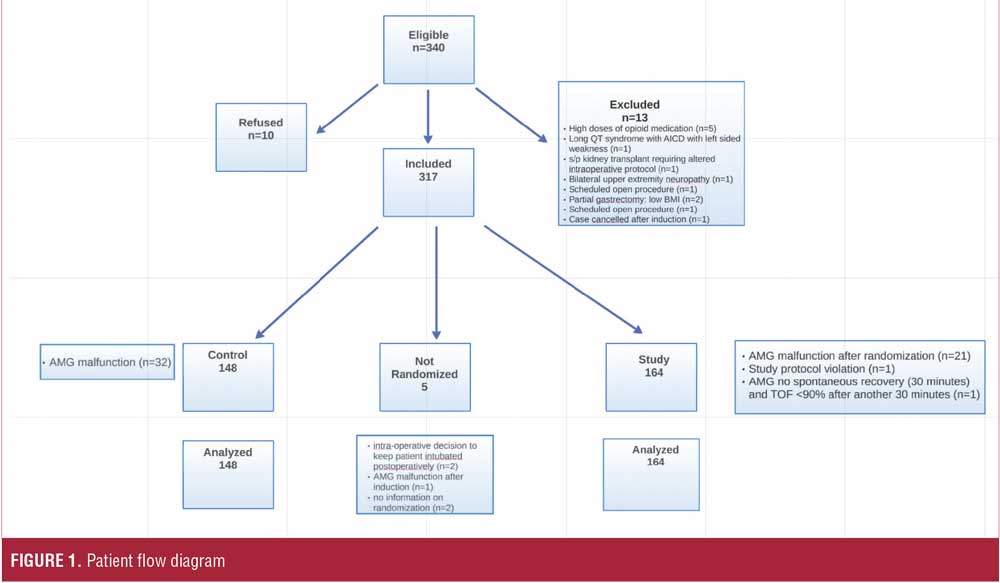
Participants. Patients were considered eligible and screened for inclusion if they were older than 18 years of age, had a body mass index (BMI) greater than 35kg/m2, and were planning to undergo elective bariatric surgery at Flagler Hospital from January 2014 to January 2016. Surgeries included laparoscopic Roux-en-Y gastric bypass (RYGB [including revision and conversion]), sleeve gastrectomy, and laparoscopic RYGB with another procedure (e.g., hiatal hernia repair). Patients with pre-existing neuromuscular disease or known allergies to any medication used in the study were excluded (Figure 1).
After written informed consent was obtained, patients were sedated with midazolam 2 to 4mg intravenous (IV) and transported to the operating room. Standard monitors and the AMG were applied. A loading dose of dexmedetomidine (0.5–1mcg/kg/hour) infused over 10 minutes was started. After preoxygenation, general anesthesia was induced with a bolus of propofol (1–2.5mg/kg/hour). After mask ventilation was established, the AMG was calibrated, and a neuromuscular blocking agent was administered (rocuronium, vecuronium, or succinylcholine). The choice and dose of neuromuscular blocking agent (NMBA) was left to the anesthesia provider’s discretion. Succinylcholine was only used to facilitate tracheal intubation and not for maintenance of neuromuscular blockade. General anesthesia was maintained with an infusion of propofol (75–200mcg/kg/min) and dexmedetomidine (0.1–0.3mcg/kg/hour). Perioperative analgesia was provided by ketamine (0.5mg/kg), paracetamol (1000mg) and ketorolac (30mg) IV.
Intraoperative administration of neuromuscular blocking agent were guided by qualitative assessment of the corrugator supercilii muscle (CS). At the end of the operation, patients were randomized (see paragraph “Randomization”) to the QuaL or QuanT group for extubation purposes.
In the QuaL group the administration of the reversal agent, neostigmine, and tracheal extubation were guided by qualitative assessment of the CS.
In the QuanT group administration of reversal agent and tracheal extubation were guided by AMG of the AP muscle. Neostigmine was administered after a spontaneous recovery to at least three twitches of the AP, and the patient’s trachea was extubated once the train-of-four (TOF) ratio ≥0.9.
It was quickly discovered after enrolling 23 patients (QuaL=11 and QuanT=12) that administration of neostigmine after spontaneous recovery to three twitches on the AMG of the AP before administration of neostigmine was insufficient to reliably reach a TOF ratio of 0.9 or greater in patients with morbid obesity. The majority of patients in the Quant group did not reach a TOF ratio of 0.9, even after waiting for 30 minutes after administration of neostigmine. This prompted a change in the protocol. Patients with morbid obesity had to show a spontaneous recovery of at least four twitches of the AP or a TOF ratio before the administration of neostigmine was permitted. The protocol in the QuaL group was not changed due to the hypothesis testing: comparing our current clinical practice using qualitative management, where we allow reversal to be administered after a TOF of three or more is observed at the CS, to the QuanT group.
After the patient’s trachea was extubated, supplemental oxygen was delivered, and the patient was transported to the postanesthesia care unit (PACU). The nurses assessed patients for respiratory symptoms documented interventions (Appendix A) and recorded the time when the patient fulfilled discharge criteria. Hydromorphone IV (0.5–1.0mg) was administered as first-line therapy for postoperative analgesia. After discharge to the ward, postoperative multimodal pain management was used.11 Paracetamol and ketorolac were given every six hours IV for the first 24 hours. For mild breakthrough pain, oral oxycodone (5–10mg) or (for severe pain) IV hydromorphone (0.5–1.0mg) was administered.
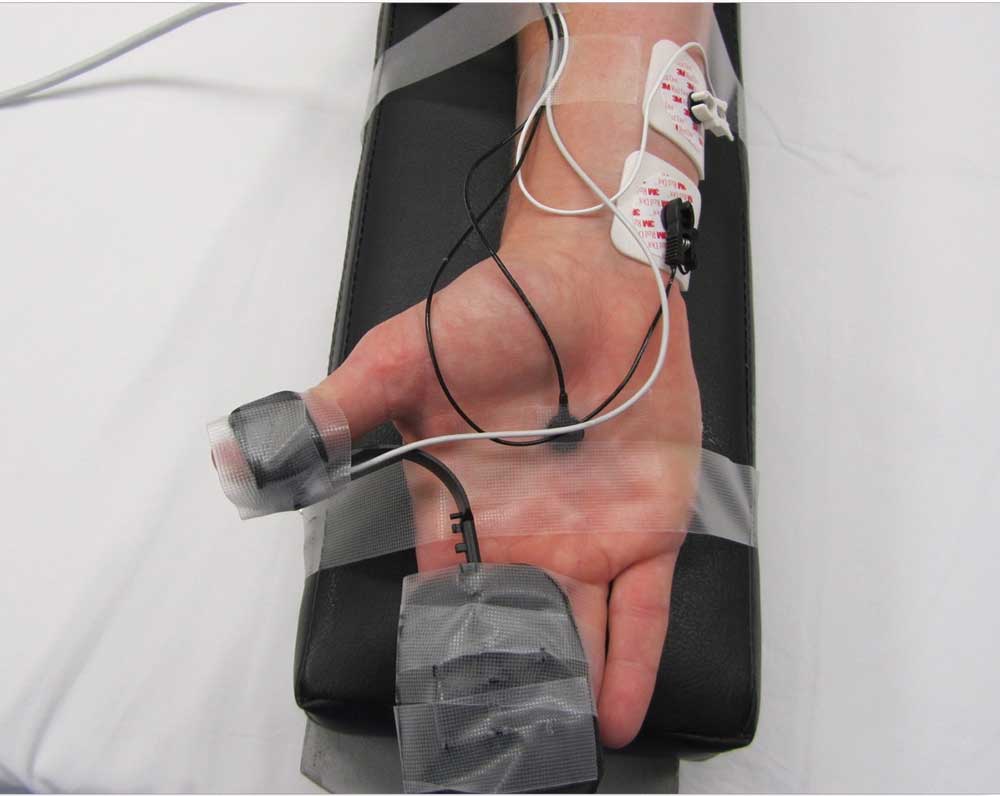
Initial setup. For quantitative monitoring of neuromuscular blockade, AMG (TOF-watch SX, Biometer, Denmark) was used. The transducer was attached to distal volar site of the thumb. The electrodes were placed above the ulnar nerve with a distance between the electrodes of 3 to 5cm. The hand was then covered with the sleeve of a forced air warming upper body blanket ensuring the thumb was able to move freely. TOF-Watch data were transferred to a computer. General anesthesia was induced and the TOF-Watch was calibrated (CAL2) to ensure supramaximal stimulation and adjustment of the sensitivity prior to administration of any neuromuscular blocking agent. If calibration was not performed (e.g., due to rapid-sequence induction, anticipated difficult airway, rapid desaturation, or parallel surgical procedures and unavailability of second TOF-Watch), the current was set at 60mA (default machine setting is 50mA). TOF stimulation was done every 15 seconds (background) and data recorded for analysis until tracheal extubation. TOF watch display, as well as the movement of the thumb, was blinded to the anesthesia provider until randomization.
Randomization. During the operation, anesthesia providers were blinded to the results of AMG monitoring (see “Initial Setup”). Patients were randomized toward the end of the surgery (trochar removal). Based on the surgical progress and the qualitative assessment measurement of a TOF of three or more, reversal could be given at this time point (“ready to reverse”). At this time, the anesthesia provider called one of the investigators to randomize, using www.random.org or R, the patients. The anesthesia provider was notified of the group randomization (QuanT/QuaL). The choice of this specific time point for randomization avoided intraoperative dosing adjustments of NMBAs based on group randomization. Providers remained blinded to their group, so as to not impact neuromuscular blocker management during the case. All dosing of NMBAs was based on qualitative assessment or surgeon request. Reversal could not be given until after group randomization.
The anesthesia providers were blinded until randomization at the end of the procedure. If a patient was randomized to the QuaL group, the anesthesia provider remained blinded to the AMG. If the patient was randomized to the QuanT group, the AMG was uncovered and used to guide the anesthesia provider in the administration of neostigmine. The PACU nurses and respiratory therapists were blinded throughout the study period. Blinded investigators performed data entry. As per protocol, unblinding was allowed in the perioperative period to allow treatment of any emergencies requiring intervention.
Definition of endpoints/respiratory symptoms and interventions. The primary endpoint in the current study was the incidence of patients developing a respiratory symptom or complication. For the definition of respiratory symptom or complication, please see Appendix A. The secondary endpoints were comparison of oxygen flows, oxygen devices, postoperative respiratory complications, the incidence of postoperative residual neuromuscular block defined as a TOF ratio below 0.9 and spirometry values in the postoperative period. Postoperative respiratory complications were defined as any new respiratory treatment or hospitalization for suspected or confirmed respiratory infection. Patients were followed either by phone interview, chart review or at the postoperative visit at two and at four weeks (Appendix B). A subgroup analysis of respiratory complications was not planned. A subgroup analysis on patients with a TOF ratio below 0.9 was not planned. A respiratory therapist (RT) assessed and examined the patient in the preoperative period. Patients were instructed to use incentive spirometry, and baseline data were recorded.
Statistical analysis. The data were entered in an Excel spreadsheet and later transferred to SPSS and Stata/IC 13.1 (StataCorp, LP, College Station, Texas) for analysis. All analyses grouped patients according to the intent-to-treat group assignment. The categorical data were analyzed with the X2 test for independence or the Fisher exact test. The quantitative data were analyzed using the unpaired Student t-test for significance. If data were not normally distributed, as assessed by the Shapiro-Wilk test, the Wilcoxon rank-sum test was used. Ordinal data were analyzed using the Wilcoxon rank-sum test. Highly skewed quantitative data were presented as median with interquartile range (IQR). Spearman correlations of ranks were used to test for trends in intraoperative characteristics over the interval of study months.
Repeated-measures data on postoperative respiratory interventions, requirement for supplemental oxygen, and presence of abnormal breath sounds were dichotomized and compared between groups using X2 tests. Repeated-measures data on incentive spirometry performance were analyzed using multivariable mixed-effects regression with a patient-level random intercept. Incentive spirometry values over the duration of hospitalization were modeled as a quadratic function of time since the earliest postoperative measurement, and were adusted for patient age, BMI, liposomal bupivacaine use, surgical time, and preoperative abnormal findings on auscultation. Intervention group differences in incentive spirometry were first assessed by introducing group assignment to the model as an intercept shift, and secondarily by interacting group assignment with the parameters of the quadratic time function. Concordance of independently evaluated AMG tracings between the investigators was confirmed using Lin’s concordance correlation coefficient. A stochastic curtailment analysis of futility for a two-sided test of two proportions was performed, assessing the likelihood of detecting the hypothesized difference in respiratory complications between the two groups if study enrollment had been completed as planned.
Power analysis for a z test difference of two independent proportions indicated that a total sample size of 362 is adequate (alpha=0.05, beta=0.2, p1=0.3, p2=0.175; G*Power 3). An incidence of 30 percent of a respiratory complication was chosen because the definition of respiratory events in the current study was more comprehensive and the observation period longer than the one used to detect an incidence of 25 percent in our institution.5 We maintain that an absolute risk reduction of 12.5 percent was clinically relevant.
The study was terminated early after enrollment of 317 patients. A futility analysis was performed to determine the conditional power rejecting the null hypothesis.
Results
Patient enrollment is shown in Figure 1. Three hundred and seventeen patients gave written consent and were included in the study. Five patients were not randomized, and those results have not been included in the analysis (see Figure 1). Reasons for not randomizing or including patients in the analysis are as follows: intraoperative decision to keep patients’ trachea intubated (n=2), AMG malfunction (n=1), and missing information on randomization (n=2). Baseline characteristics are presented in Table 1.
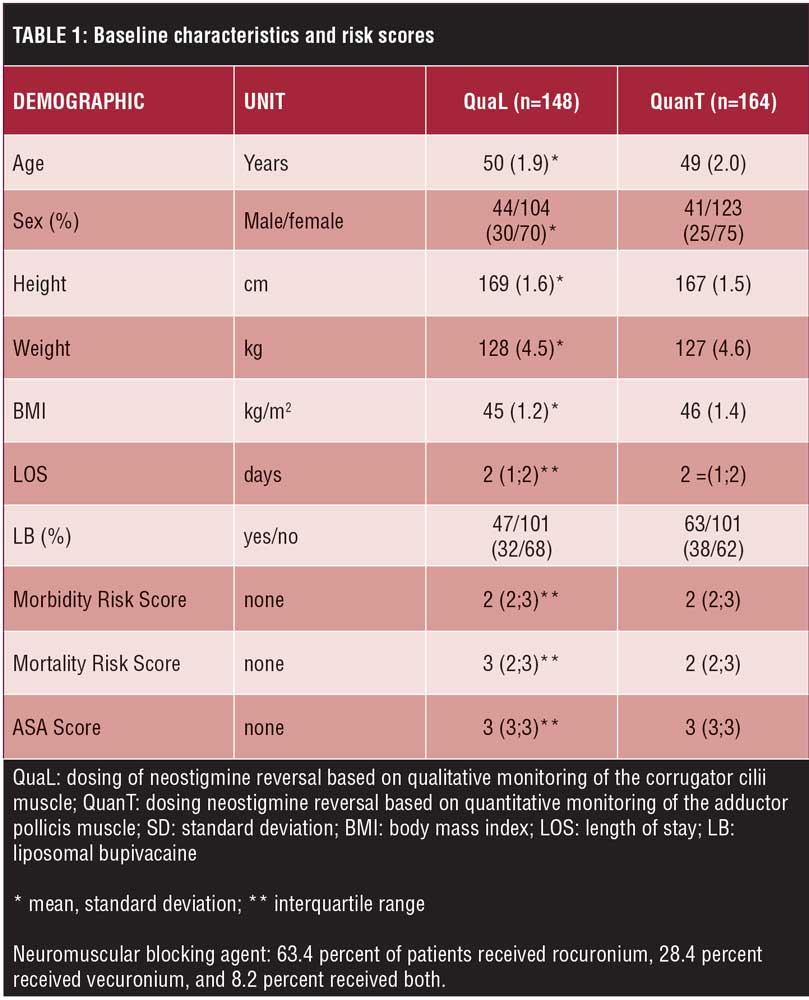
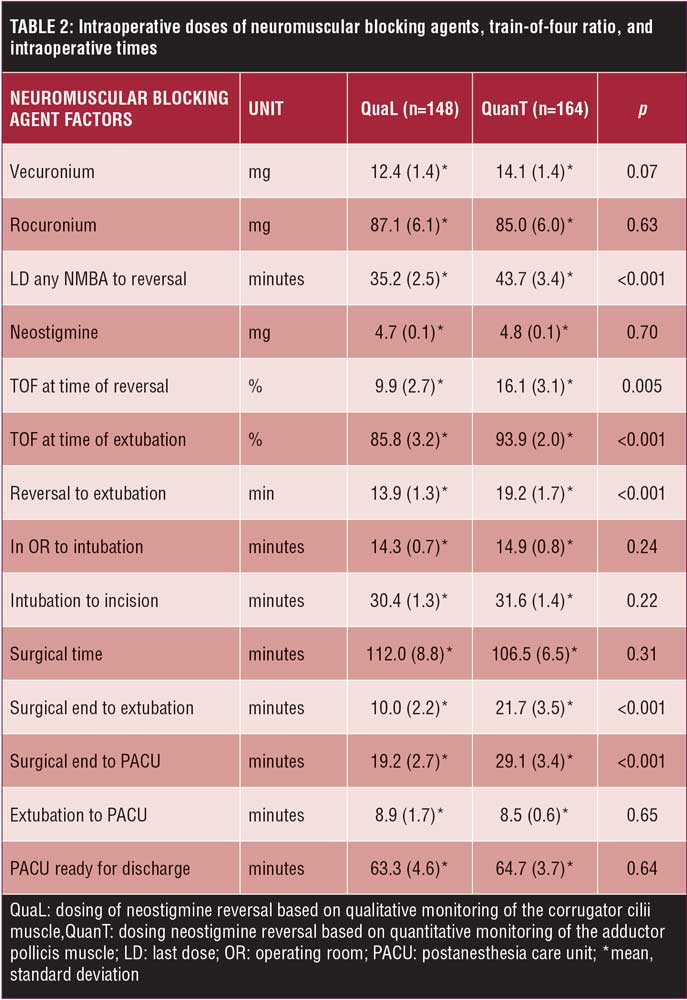
Intraoperative. In total, 63.4 percent of patients received rocuronium, 28.4 percent received vecuronium, and 8.2 percent received both. There was no difference in the total doses of NMBAs or neostigmine. The time interval from the last dose of NMBA to reversal was longer by approximately eight minutes in the QuanT group relative to the QuaL group (Table 2). TOF at the time of reversal and at the time of extubation was significantly lower in the QuaL group relative to the QuanT group (Table 3). The Lin’s concordance correlation coefficient demonstrated strong agreement of the TOF ratio between the two investigators at the time of reversal (pC=0.70, p<0.001) and at the time of extubation (pC=0.92, p<0.001). Furthermore, the time after reversal to extubation was significantly shorter in the QuaL group compared to the QuanT group. This extends the time from surgical end to extubation to a similar degree (Table 2). There were no statistically significant trends in the timing or dose of NMBA used over the two-year study period. More patients in the QuaL group had postoperative residual neuromuscular block compared to the QuanT group, TOF ratio 0.537 versus 0.231, respectively (p<0.001).
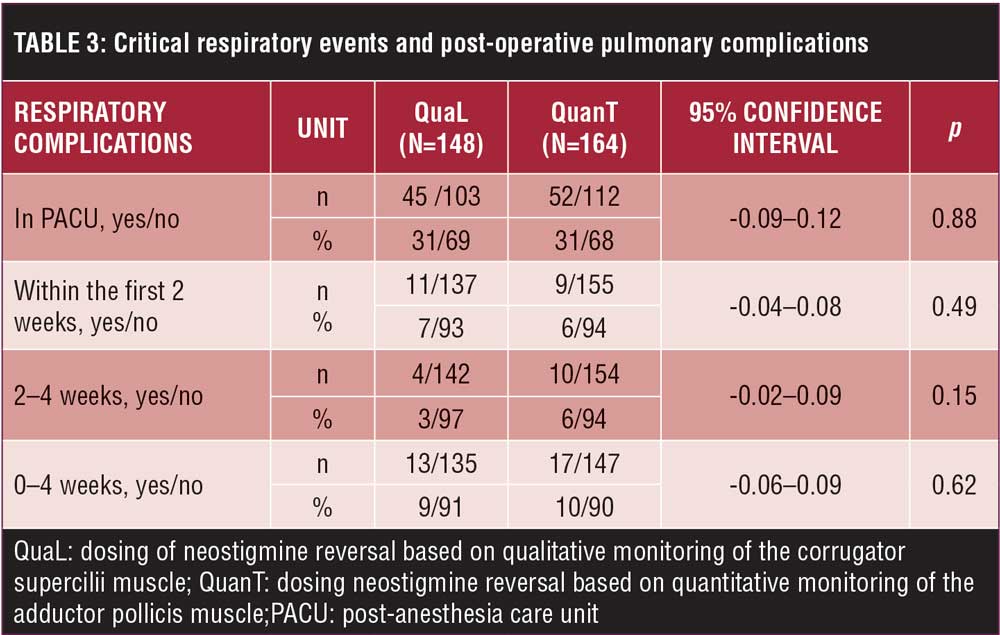
PACU. Hemoglobin oxygen saturation, oxygen flow, and device did not differ on PACU arrival. On PACU discharge, patients in the QuanT group required a higher flow of oxygen with similar oxygen saturation using nasal cannula (Table 2). There was no difference in the number of patients having respiratory complications (p=0.88) (Table 3).
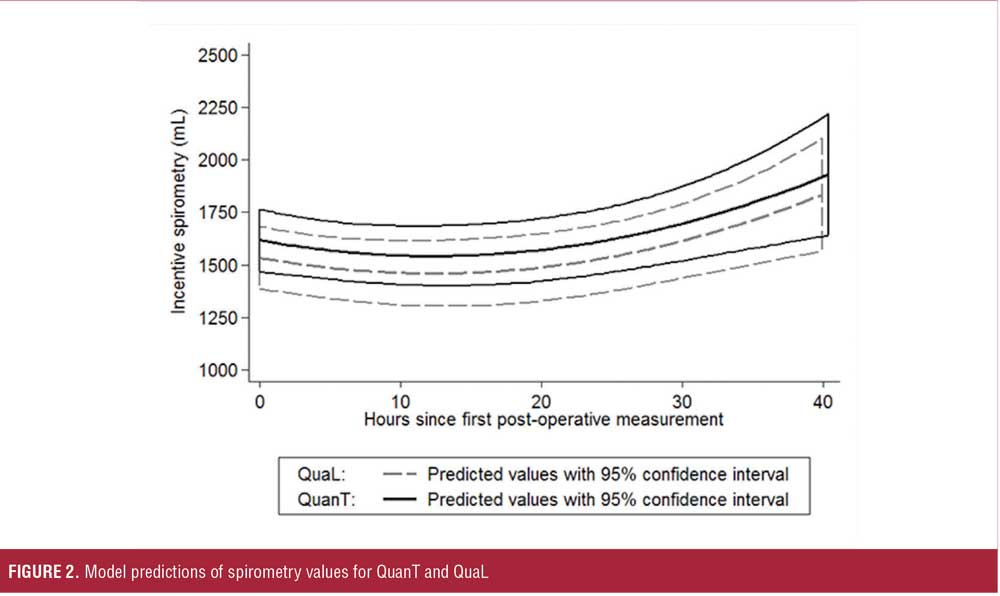
Postoperative. There was no difference in the number of patients requiring treatment from RTs (QuanT: 13%; QuaL: 14%) or requiring an oxygen device (QuanT: 58%; QuaL: 68%) in the postoperative period. The lowest hemoglobin oxygen saturation was similar in both groups (QuanT: 96±2; QuaL: 96±2). There was also no difference in patients having abnormal findings on auscultation (QuanT: 76%; QuaL: 74%). Mixed-effects regression of spirometry values over the initial (40-hour) postoperative period found no difference in this outcome between QuanT and QuaL when group assignment was considered to have an equal effect on spirometry at each time point (intercept shift of 83mL; 95% confidence interval [CI]: -121, 287; p=0.425). Model predictions of spirometry values for QuanT and QuaL are shown in Figure 2. Further analysis found that group assignment did not significantly modify the shape of the spirometry trajectory over the first two days of hospitalization. There was also no difference in the number of patients suffering from a respiratory complication in the first four weeks after hospital discharge at any time point (p=0.62 [Table 3]).
Because of early termination of the study, a futility analysis was performed to assess the likelihood of detecting the hypothesized difference in respiratory complications between the two groups, if study enrollment had been completed as planned. Given the observed data and expected effect size, the study had less than one percent conditional power for rejecting the null hypothesis if the target enrollment would have been achieved.
Discussion
Qualitative management of neuromuscular blockade leaves patients with morbid obesity undergoing bariatric surgery with a mild degree of residual neuromuscular block.
There are several possible reasons for not detecting a difference in respiratory complications between groups. First, there was only a small degree of difference in residual neuromuscular block in the QuaL group compared to the QuanT group. The current recommendation based on expert opinion is to extubate the patient’s trachea after TOFr greater than 0.90 measured quantitatively at the adductor pollicis.3 In previous studies, patients who developed a respiratory complication had a lower TOF ratio compared to the current study. In a case-control study where 2 out of 3 patients could be matched to controls, respiratory complications (hypoxemia and upper airway obstruction) were more common in patients with postoperative residual neuromuscular block compared to controls (TOF 0.62 versus 0.98).9 In a prospective trial, mild hypoxemia (SaO2 90–93%) and swallowing difficulties were more common in patients with postoperative residual neuromuscular block (TOF 0.7 versus 1.0).10 In the current study, however, patients in the QuaL group had only a mild degree of residual block compared to the QuanT group (TOFr 0.86 versus 0.94). It is worth mentioning that AMG measurements suffer from frequent technical problems and are in uncooperative patients fairly unreliable during emergence from anesthesia.11 The patients remained unconscious (motionless) under propofol anesthesia in the QuanT group, until several reliable TOF measurements of more than 90 percent were achieved. Still, a total of 53 (16.9%) TOF-watch tracings could not be analyzed because of technical problems. In QuanT group 21 (12.9%) patients had to be reversed and extubated based on the qualitative method of monitoring neuromuscular blockade. Data were analyzed using the intention-to-treat method, and these patients remained in the QuanT group.
Another reason for not detecting a difference of respiratory complications could have been the exclusive use of intermediate NMBAs. In a prospective, randomized trial, residual neuromuscular block from intraoperative pancuronium use increased the risk of developing a postoperative pulmonary complication (POPC). Residual neuromuscular block in patients treated with intermediate acting NMBA (vecuronium/atracurium) did not show an increased risk of POPC.4 In data mining studies,12,13 high doses of NMBAs and neostigmine were associated with an increase in respiratory complications. Data mining has its limitations,14 and, in the current prospective, randomized trial, equal doses of intermediate acting NMBAs (vecuronium and rocuronium) and neostigmine were used in both groups.
Furthermore, the longer time of tracheal intubation and ventilation in the QuanT group and the use of supplemental oxygen could have reduced the difference of respiratory complications between groups. In a prospective study by Sauer et al,10 residual block increased the risk of mild hypoxemia (90–93%) but did not affect the risk of severe hypoxemia (<90%). Oxygen is used routinely for transport in our institution. During the hospital stay, oxygen saturations were similar in both groups. In the QuanT group, patients were intubated and ventilated longer compared to the QuaL group. This could have, theoretically, increased the severity of pulmonary atelectasis, elevating oxygen needs in the QuanT group.
During the study planning, we expected to find a lower TOF ratio, more patients with post-operative residual neuromuscular block, and more respiratory complications in the QuaL group compared to the QuanT group for mainly two reasons: 1) patients with morbid obesity are considered a high-risk patient population and 2) the method/location of monitoring the degree of paralysis.
Patients with morbid obesity are at high risk for respiratory events5 and atelectasis15 after bariatric surgery. Previous studies excluded patients with morbid obesity.4,10 In the study by Murphy et al,9 patients underwent a variety of different surgical procedures and had BMIs of approximately 30kg/m2. In our study, all patients with morbid obesity (BMI≈45kg/m2) underwent bariatric (upper abdominal) operations.
Quantitative (AMG) compared to qualitative methods of monitoring have been shown to be superior in detecting recovery from neuromuscular blockade at the end of operation. Qualitative methods frequently fail to detect TOF >0.41.16 In the QuaL group, the CS was used to monitor the degree of neuromuscular blockade. The CS is more resistant to the effect of NMBA than the AP.17 Therefore, using a method (qualitative) that is imprecise to detect recovery from neuromuscular blockade and more resistant muscle group (CS) to the effect of NMBAs should have left patients with a higher degree of postoperative residual neuromuscular block—a greater degree of block at other muscles including respiratory.
A limitation of the current study is the early termination. It was terminated because sugammadex became available at our institution. The investigators felt it would be unethical to withhold a superior drug from study participants. An insufficient number of patients were enrolled according to the power analysis during study planning. However, futility analysis indicated less than one percent conditional probability of rejecting the null hypothesis for the primary outcome if the intended number (n=362) of patients had been enrolled. Another limitation affecting the validity of the current study is that the protocol had to be changed in the QuanT group. The protocol allowed reversal to be administered in the QuanT as soon as at least three twitches of the TOF were detected on the AMG at the AP. Patients with morbid obesity enrolled in the study that received neostigmine at a TOF of three twitches did not reliably recovere to a TOF ratio of more than 0.9. The decision was made to delay administration of neostigmine until a TOF of four twitches or a TOF ratio was detected.
In conclusion, qualitative compared to quantitative management of neuromuscular blockade in patients with morbid obesity during bariatric surgery leads to a mild degree of postoperative residual neuromuscular block. This mild degree of postoperative residual neuromuscular block does not seem to increase the risk for critical respiratory events.
Acknowledgments
The authors wish to thank respiratory therapists Nancy Hammert, Edwin Soto, Jaceline Hynjub, Allen Williams as well as Mike DiBella, MD, and Pamela Barberi, DNP, of Flagler Hospital (St. Augustine, Florida) for their outstanding support and effort to facilitate this study. We also wish to thank Sorin Brull, MD, of Mayo Clinic in Jacksonville, Florida, for giving insightful guidance with study design, and to Casper Claudius, MD, PhD, (Department of Intensive Care, Copenhagen University Hospital, Copenhagen, Denmark) for reviewing the tracings and the manuscript. We would like to extend our gratitude to Jana Barlic-Dicen, PhD, for reviewing the manuscript.
References
- Smetana GW, Lawrence VA, Cornell JE, American COP. Preoperative pulmonary risk stratification for noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med. 2006;144:581–595.
- Canet J, Gallart L. Predicting postoperative pulmonary complications in the general population. Curr Opin Anaesthesiol. 2013;26:107–115.
- Brull SJ, Murphy GS. Residual neuromuscular block: lessons unlearned. Part II: methods to reduce the risk of residual weakness. Anesth Analg. 2010;111:129–140.
- Berg H, Viby-Mogensen J, Roed J, et al. Residual neuromuscular block is a risk factor for postoperative pulmonary complications. A prospective, randomised, and blinded study of postoperative pulmonary complications after atracurium, vecuronium and pancuronium. Acta Anaesthesiol Scand. 1997;41:1095–1103.
- Ziemann-Gimmel P, Hensel P, Abdo S, et al. Respiratory events in patients undergoing laparoscopic gastric bypass surgery. F1000 Research. 2012;1–46
- Caffrey RR, Warren ML, Becker KE. Neuromuscular blockade monitoring comparing the orbicularis oculi and adductor pollicis muscles. Anesthesiology. 1986;65:95–97.
- Thilen SR, Hansen BE, Ramaiah R, et al. Intraoperative neuromuscular monitoring site and residual paralysis. Anesthesiology. 2012;117:964–972.
- Donati F. Neuromuscular monitoring: more than meets the eye. Anesthesiology. 2012;117:934–936.
- Murphy GS, Szokol JW, Marymont JH, et al. Residual neuromuscular blockade and critical respiratory events in the postanesthesia care unit. Anesth Analg. 2008;107:130–137.
- Sauer M, Stahn A, Soltesz S, et al. The influence of residual neuromuscular block on the incidence of critical respiratory events. A randomised, prospective, placebo-controlled trial. Eur J Anaesthesiol. 2011;28:842–848.
- Baillard C, Bourdiau S, Le Toumelin P, et al. Assessing residual neuromuscular blockade using acceleromyography can be deceptive in postoperative awake patients. Anesth Analg. 2004;98:854–857, table of contents.
- Grosse-Sundrup M, Henneman JP, Sandberg WS, et al. Intermediate acting non-depolarizing neuromuscular blocking agents and risk of postoperative respiratory complications: prospective propensity score matched cohort study. BMJ. 2012;345;e6329.
- McLean DJ, Diaz-Gil D, Farhan HN, et al. Dose-dependent association between intermediate-acting neuromuscular-blocking agents and postoperative respiratory complications. Anesthesiology. 2015;122:1201–1213.
- Kopyeva T, Sessler DI, Weiss S, et al. Effects of volatile anesthetic choice on hospital length-of-stay: a retrospective study and a prospective trial. Anesthesiology. 2013;119:61–70.
- Eichenberger A, Proietti S, Wicky S, et al. Morbid obesity and postoperative pulmonary atelectasis: an underestimated problem. Anesth Analg. 2002;95:1788–1792.
- Viby-Mogensen J, Jensen NH, Engbaek J, et al. Tactile and visual evaluation of the response to train-of-four nerve stimulation. Anesthesiology. 1985;63:440–443.
- Hemmerling TM, Le N. Brief review: Neuromuscular monitoring: an update for the clinician. Can J Anaesth. 2007;54:58–72.
Category: Original Research, Past Articles



