Perforated Ulcer in the Gastric Remnant after Roux-en-Y Gastric Bypass Presenting as Acute Cholecystitis

by Khea Tan, MD; Jason Silvers, MD; and Seth Kipnis, MD
All authors are with the Department of Surgery, Jersey Shore University Medical Center in Neptune, New Jersey.
Funding: No funding was provided for this article.
Disclosures: The authors have no conflicts of interest relevant to the contents of this article.
Correspondence: Khea Tan, MD; Email: [email protected]
Bariatric Times. 2023;20(7–8). Published online July 21, 2023.
Abstract
We present the case of a 63-year-old female patient who presented with several months of upper abdominal pain, with history of Roux-en-Y gastric bypass (RYGB) 12 years prior. She had imaging suggestive of cholecystitis and inflammation surrounding the gastric remnant. Diagnostic laparoscopy and cholecystectomy were performed, and she was found to have a previously perforated ulcer in the gastric remnant with a sealed-off abscess cavity that was then drained. Perforations of the gastric remnant are rare complications that usually occur years after RYGB, and pneumoperitoneum is rarely seen; however, clinicians must remain astute when evaluating for gastric remnant pathology, and surgery should not be delayed.
Keywords: Roux-en-Y gastric bypass, perforated ulcer, endoscopy, cholecystitis
Roux-en-Y gastric bypass (RYGB) is widely performed as a treatment for obesity. However, due to the reconstructed anatomy, it can be difficult to accurately diagnose complications of RYGB, especially those associated with the gastric remnant. Oftentimes, these diagnoses can manifest in other ways, or without pneumoperitoneum, as typically seen in perforated hollow viscus injuries. There are a limited number of cases described in the literature of perforation of the remnant stomach after RYGB, and we present a case of perforated ulcer of the bypassed stomach presenting as cholecystitis.
Case Report
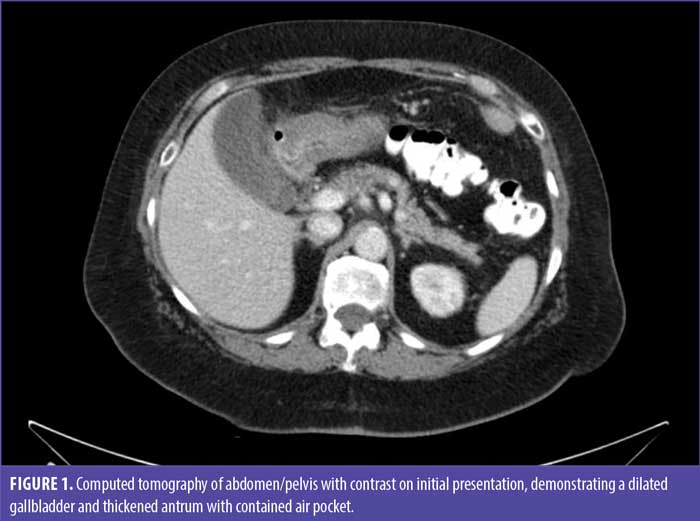
We present the case of a 63-year-old female patient with a history of RYGB 12 years prior to admission who presented with intermittent upper abdominal pain lasting several months, eventually localizing to the right upper quadrant. This pain was associated with nausea and dry heaves that were exacerbated with food intake. Her initial bypass was complicated by anastomotic stricture one-month postoperatively; however, following dilation, she had no other issues. Computed tomography (CT) of the abdomen and pelvis with oral and intravenous contrast was obtained, which showed a distended gallbladder, small ascites over the right upper lobe of the liver and around the excluded pylorus extending to the gallbladder, thickened gastric remnant, and a contained foci of air near the antrum (Figure 1). Abdominal ultrasound was then performed, which was consistent with cholecystitis. Labs, including white blood count, liver function tests, and lactate, were within normal limits. Our differential diagnosis included cholecystitis, as well as marginal ulceration. An esophagogastroduodenoscopy (EGD) was performed, failing to identify a marginal ulcer and demonstrating a normal-appearing gastric pouch.
We discussed these findings with the patient, and the decision was made to perform a cholecystectomy and diagnostic laparoscopy to evaluate the gastric remnant. Upon inspection of the abdomen, the alimentary limb appeared normal. The gallbladder was noted to have a thick inflammatory rind along the region adjacent to the antrum. The pylorus had fibrinous exudate, which was peeled back to reveal an abscess cavity that was drained and sent for culture. The gallbladder was removed. A purse string suture was placed around healthy gastric tissue, and a gastrotomy was made to evaluate the gastric remnant with endoscopy. The endoscope was placed through a 12mm trocar, and the suture ends were pulled up and tightened around the endoscope to facilitate insufflation of the stomach. The remnant stomach appeared normal and blind-ending, and there were no signs of masses or ulcerations; however, friable tissue was noted distal to the gastrotomy. Biopsies of this area were taken, the gastrotomy was closed with a purse string suture, and a drain was placed in the area of the abscess cavity. Final pathology of the biopsy was negative for malignancy and was consistent with gastric tissue. Gallbladder pathology was consistent with chronic cholecystitis, cholelithiasis, and inflamed granulation tissue. Postoperatively, the patient recovered without complications. She has been maintained on long-term proton pump inhibitors (PPI) and counseled to avoid nonsteroidal anti-inflammatory drugs (NSAIDs).
Discussion
RYGB is a commonly performed operation to address obesity in the United States (US), accounting for 41,280 out of 198,000 bariatric surgeries in 2020.1 A common complication of bariatric surgery is gallstone formation, and approximately 32 to 50 percent of patients will develop gallstones or sludge; however this usually does not warrant concomitant cholecystectomy at the time of surgery.2,3 Gallstone formation following bariatric surgery has been speculated to be related to excretion of cholesterol from fatty tissue into bile following rapid weight loss or to an increased prostaglandin and arachidonic acid concentration due to gallbladder secretion of mucin and calcium.2
A rare complication of RYGB is perforation of the gastric remnant. This has been described a handful of times in the literature, and presentation can vary in time from days to years following surgery.4 Imaging often does not show pneumoperitoneum, possibly because the excluded stomach does not contain swallowed air, although if free air is present, it might be due to reflux from the alimentary limb.5 More importantly, the presence of ascites should alert to the possibility of perforation in the bypassed limb. Bjorkman et al6 suggested that the remnant pouch does not benefit from the neutralizing effects of ingested food and therefore remains subject to acidification from the stomach and duodenum. There is also a delay in bicarbonate secretion, as the normal stimulus for pancreatic secretion is bypassed after RYGB.
The same risk factors for marginal ulcer formation apply to ulceration of the gastric remnant. These include a history of smoking, immunosuppression, Helicobacter pylori infection, and preoperative NSAID use.7 Postoperatively, patients should be on lifelong PPI therapy and counseled to avoid these risk factors to prevent mucosal injury.
In this patient, the perforation likely sealed off naturally, as there was no evidence of recent perforation on endoscopic evaluation. The presence of free fluid near the gallbladder likely incited persistent inflammation, leading to her symptoms of upper abdominal pain. Though rare, clinicians should consider perforation of the gastric remnant as a differential diagnosis, even without pneumoperitoneum, especially when free fluid is present. An EGD should also be performed to evaluate for marginal ulcers. Endoscopic evaluation of the remnant is more difficult as a result of Roux-en-Y anatomy, but it can be done using laparoscopy and anterior gastrotomy to evaluate for malignant causes of perforation and obtain biopsies. In scenarios where perforation is identified, these can be repaired using an omental patch in small perforations less than two centimeters in diameter or those presenting in shock. Larger or more complex perforations can be repaired primarily; however, this requires healthy tissue, minimal tension, and omental reinforcement.8 When primary repair is not feasible or the perforation is too large to be covered with an omental patch, gastrectomy with gastrojejunostomy reconstruction can be considered, but the literature is inconclusive on this approach, with some studies suggesting that gastrectomy does not increase morbidity or mortality, compared to primary repair or omental patch.9
Conclusion
It is important to keep these rare complications in mind when evaluating a patient with history of RYGB, as they can be easily missed. Surgery should not be delayed in order to reduce morbidity and mortality in these patients.
References
- American Society for Metabolic and Bariatric Surgery. Estimate of bariatric surgery numbers, 2011–2020. 27 Jun 2022. https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers. Accessed 26 Jul 2022.
- Villegas L, Schneider B, Provost D, et al. Is routine cholecystectomy required during laparoscopic gastric bypass? Obes Surg. 2004;14(1):60–66.
- Jonas E, Marsk R, Rasmussen F, Freedman J. Incidence of postoperative gallstone disease after antiobesity surgery: population-based study from Sweden. Surg Obes Relat Dis. 2010;6(1):54–58.
- Macgregor AM, Pickens NE, Thoburn EK. Perforated peptic ulcer following gastric bypass for obesity. Am Surg. 1999;65(3):222–225.
- Zagzag J, Cohen NA, Fielding G, et al. Lack of diagnosis of pneumoperitoneum in perforated duodenal ulcer after RYGB: a short case series and review of the literature. Obes Surg. 2018;28(9):2976–2978.
- Bjorkman DJ, Alexander JR, Simons MA. Perforated duodenal ulcer after gastric bypass surgery. Am J Gastroenterol. 1989;84(2):170–172.
- Di Palma A, Liu B, Maeda A, et al. Marginal ulceration following Roux-en-Y gastric bypass: risk factors for ulcer development, recurrence and need for revisional surgery. Surg Endosc. 2020;35(5):2347–2353.
- Mouly C, Chati R, Scotté M, Regimbeau J-M. Therapeutic management of perforated gastro-duodenal ulcer: literature review. J Visc Surg. 2013;150(5):333–340.
- Zhu C, Lin A, Mathur N, et al. Gastric resection vs. omental patch for perforated gastric ulcers: systematic review and meta-analysis for an unresolved debate. J Am Coll Surg. 2020;231(4):e127–e127.
Category: Case Report, Online Only




