Perioperative Glycemic Control for the Patient with Type 2 Diabetes Receiving Bariatric Surgery
by Teri Leishman, MS, PMC, APRN-CNS, AGCNS-BC, CPAN, CAPA
Ms. Leishman is a Clinical Nurse Specialist with Intermountain Health, Peaks Region, St. Mary’s Medical Center in Grand Junction, Colorado.
Funding: No funding was provided for this article.
Disclosures: The author reports no conflicts of interest relevant to the content of this article.
Bariatric Times. 2022;19(11):8–10.
Abstract
Objective: This evidence-based practice (EBP) initiative sought to improve perioperative glucose management by developing a quality improvement protocol to guide EBP at the bedside.
Design: Evidence-based practice/quality improvement.
Setting and Participants: Our organization is a 350-bed level two trauma center in western Colorado. Inclusion criteria are patients with T2DM completing bariatric surgery.
Measurements: Primary outcomes were postoperative BG levels and secondary outcomes included intra-operative dexamethasone dosing.
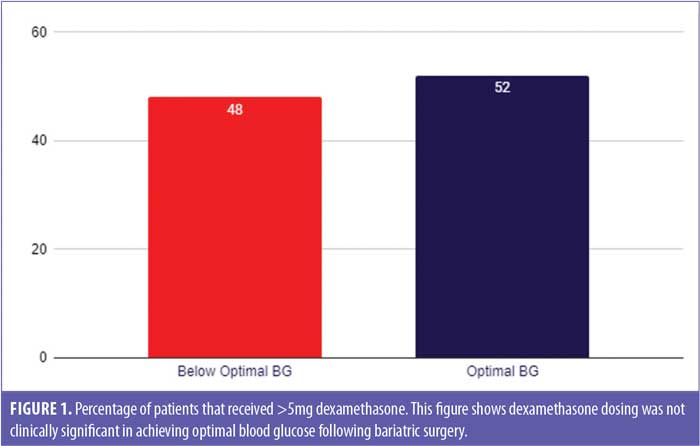
Outcomes: Between October 2021 and July 2022 optimal BG increased by 49 percent after implementing an insulin protocol. Dexamethasone dosing was not clinically significant in postoperative BG levels in this population.
Conclusion: Use of an insulin protocol can improve perioperative glucose management. This has important implications for practice when we consider the comorbidity reductions following bariatric surgery extends the life expectancy of patients with diabetes by over 9 years and diabetes resolution alone correlates to a $5.4 million dollar savings for every 1000 surgical patients. These outcomes are likely to increase the use of bariatric surgery as a public health measure to reduce the twin epidemics of obesity and diabetes.
Keywords: Bariatric surgery, metabolic surgery, stomach stapling, perioperative care, perioperative period, insulin, regular insulin, soluble insulin, diabetes mellitus, prediabetes, protocol, glucose intolerance, gastroparesis, insulin resistance
Multiple high-quality research studies have confirmed that patients with obesity have an increased risk of all-cause mortality. In 2019, obesity contributed to 160 million disability-adjusted life years and 5 million deaths.1 Obesity has been added to the global noncommunicable disease targets by the World Health Organization (WHO), and according to the National Health and Nutrition Examination Survey that was conducted between 2013 and 2016, nearly 40 percent of adults in the United States (US) and 19 percent of youth aged 2 to 19 years have obesity, and the Centers for Disease Control and Prevention (CDC) estimates that 15 percent of Americans have severe obesity, with a body mass index (BMI) greater than 35kg/m2.2,3
Obesity and Diabetes
The complications of obesity are well known and increase the risks of developing multiple chronic diseases, such as cardiovascular disease, metabolic syndrome, nonalcoholic fatty liver disease (NAFLD), multiple cancers, and diabetes.3 Obesity and diabetes have often been described as twin epidemics. Since 2013, there has been a substantial body of evidence published suggesting bariatric surgery significantly improves mortality and reduces the risks of developing chronic diseases, particularly benefiting patients with Type 2 diabetes (T2D) and prediabetes.2
Bariatric surgery as a treatment for Type 2 diabetes. In 2016, the 2nd Diabetes Surgical Summit4 was convened in collaboration with representatives from several leading organizations to develop guidelines and inform clinicians about the benefits and risks of bariatric surgery for T2D treatment. These guidelines included the addition of recommending bariatric surgery to its algorithm for patients with T2D and a BMI greater than 35kg/m2 and recommending the consideration of bariatric surgery for patients with T2D and a of BMI 30 to 34.9kg/m2. These recommendations are reinforced by the American Diabetes Association (ADA) obesity management for the treatment of T2D standards of medical care.5 When blood glucose (BG) is optimized during bariatric surgery, many patients with T2D experience a complete resolution of their diabetes within one year.6,7 Bariatric surgery extends the median life expectancy by 5.1 years in patients without diabetes and 9.3 years for patients with pre-existing diabetes.2 These changes are likely to increase the use of bariatric surgery as a public health strategy.
Perioperative Glucose Management and Evidence-based Practice
High reliability organizations utilize safety checklists and protocols to improve patient safety. The American Diabetes Association (ADA) recommends the use of a best practice protocol when providing care to hospitalized diabetes patients.8 Rarely do bariatric surgery patients achieve optimal BG levels without the use of a standardized protocol.6,7,9
In 2019, our organization implemented enhanced recovery after surgery (ERAS) in this population, including preoperative carbohydrate loading. Carbohydrate loading with 50g of carbohydrates has been shown to reduce postoperative insulin resistance.10,11 While evidence suggests it is safe to carbohydrate load patients with T2D,12–14 high quality studies are lacking. It is our organization’s practice to carbohydrate load patients with T2D.
Historically, our organization did not utilize a standardized insulin protocol in the perioperative phases of care. This has resulted in 55 percent of our bariatric surgery patients achieving optimal BG measurements in the first 24 hours following surgery (Figure 1). To explore practice improvement opportunities, the following patient, intervention, comparison, outcome and time (PICOT) question was developed: In adult bariatric surgery patients with T2D (P) does the use of a perioperative insulin protocol (I) versus not using a protocol (C) provide better stability of BG levels (O) in the first 24 hours (T) following surgery?
Literature Review
The following keywords and medical subject headings (MeSH) terms were used in the literature search: “bariatric surgery,” “bariatric surgeries,” “metabolic surgery,” “metabolic surgeries,” “bariatric surgical procedure,” “stomach stapling,” “perioperative care,” “perioperative period,” “insulin,” “regular insulin,” “soluble insulin,” “diabetes mellitus,” “prediabetes,” “protocol,” “glucose intolerance,” “gastroparesis,” and “insulin resistance.” Boolean phrases “and” or “or” were used to connect keywords and MeSH terms to narrow or broaden the search as needed. PubMed, CINAHL, Google Scholar, and Cochrane Database were utilized.
The inclusion criteria included English language, peer-reviewed journals between 2016 and 2021, including serum glucose management, bariatric surgical population, and the perioperative phases of care. The first search resulted in 91 abstracts that were surveyed for appropriateness and reduced to 21 articles for appraisal. The 91 abstracts were then filtered to guidelines and randomized, controlled trials (RCTs), which resulted in 15 RCT abstracts, most of which were not specific to bariatric or surgery and zero guidelines. The search for guidelines was expanded to 20 years, which resulted in seven articles that were then surveyed and reduced to three hyperglycemic guideline examples. Selected for the literature review were one RCTx, one quality improvement study, one retrospective study, two expert opinion articles, and one informative literature review.
Polderman et al9 conducted an RCT to compare three perioperative glucose management protocols. Patients were randomly assigned to either a protocol that used the glucagon-like peptide-1 (GLP-1) agonist, liraglutide, a second protocol that used an insulin infusion, or a third that used an insulin bolus. They reported that in the perioperative period, BG peaks one hour following surgery. The aim of this study was to determine which of the three protocols best prevented this first hyperglycemic spike. The study found that all three protocols met the goal of preventing hyperglycemia one hour following surgery without episodes of hypoglycemia. Liraglutide was the most effective at preventing insulin requirements, compared to the other two methods of insulin delivery (p=0.006). However, several patients suffered from preoperative nausea, and two had severe nausea. None of the patients in either insulin group experienced nausea.9
Ehrenfeld et al15 conducted a quality improvement study that leveraged technology to reduce variability and ensure consistent, standardized performance by automating their perioperative glucose management protocol. An automated system was created to identify patients with diabetes, track insulin administration and BG measurements, and remind anesthesiologists when it was time to check patient BG inside the operating room (intraoperatively). They compared preintervention cases with postintervention cases that were then propensity score matched. Intraoperative BG monitoring rose from 62 to 87 percent (p=0.0001) following the interventions. Postanesthesia care unit (PACU) hyperglycemia dropped from 11 to 7 percent, while hypoglycemia remained unchanged. There was also a significant drop in surgical site infections during the study that equated to a 55.4-percent relative risk reduction. Interestingly, nearly 30 percent of patients with diabetes were not appropriately flagged prior to surgery.15
The American Society for Metabolic and Bariatric Surgery (ASMBS) defines optimal glucose control as all point-of-care glucose readings immediately postoperative to discharge less than 180mg/dL. Zaman et al6 conducted a retrospective review of 155 cases of patients who underwent bariatric surgery to investigate 90-day and one-year outcomes, including diabetes resolution, of optimal perioperative glucose control versus nonoptimal glucose control in the perioperative phases of care. Patients underwent either laparoscopic Roux-en-Y gastric bypass (RYGB) or sleeve gastrectomy (LSG). The authors concluded that complication rates, emergency room (ER) visits, readmissions, length of stay, and blood transfusions were not significantly different between the two groups. However, optimal BG control in the perioperative phases was highly predictive of diabetes resolution at one year, with 97 percent of the optimally controlled group achieving complete resolution, compared to only 53 percent (p<0.001) of the nonoptimally controlled group reaching complete diabetes remission at the one-year mark.6
In 2017, Duggan et al16 noted a clear association between perioperative hyperglycemia and adverse clinical outcomes. Their article describes the prevalence, and pathophysiology of perioperative hyperglycemia, along with providing a practical outline to manage surgical patients in the perioperative phases of care. This group defined hyperglycemia as a BG greater than 180mg/dL and reported a prevalence in both intensive care unit (ICU) and non-ICU patients of 32 percent. These authors reported that one-year mortality is significantly related to preoperative BG in noncardiac surgeries and that long-term outcomes were not worse with a liberal BG management goal of 120 to 180mg/dL, compared to 90 to 120mg/dL. These authors report that many organizations, such as the Society for Ambulatory Anesthesia (SAMBA), the American Association of Clinical Endocrinologists (AACE), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons all recommend BG be maintained at less than 180mg/dL, and the American College of Surgeons (ACS) recommends targets of 140 to 200mg/dL. They also suggested that procedures less than four hours in duration are appropriate for subcutaneous injections, and in procedures that are greater than four hours, an insulin infusion is recommended, with regular two-hour BG checks in both cases. The authors agreed with the large Randomized Study of Basal-Bolus Insulin Therapy in the Inpatient Management of Patients with Type 2 Diabetes Undergoing General Surgery (RABBIT-2) study that determined basal plus bolus results in better glycemic control in patients with diabetes following surgery; however, this study was not specific to bariatric surgery. The use of insulin pumps and continuous glucose monitoring has gained popularity in both Type 1 and T2D. These authors recommended hourly glucose checks if insulin pumps remain on at a reduced rate during surgery. They concluded that multidisciplinary groups should work together to develop appropriate protocols for hyperglycemia screening and treatment during the perioperative phases.16
Specifically looking at the bariatric surgery population, Kheniser and Kashyap17 aimed to provide recommendations for diabetes management before, during, and after bariatric surgery. These authors recommended a target glucose range between 140 to 180mg/dL and use of subcutaneous insulin during the perioperative phases of care. They reported that a one-size-fits-all approach ot postoperative glucose management in the bariatric surgery population is not feasible. They also suggested discontinuing basal insulin if it is less than 30 units prior to surgery and using a regular sliding scale insulin protocol (SSI) to cover postprandial BG. If the patient is on greater than 30 units of basal insulin prior to surgery, the dose should be reduced by 50 to 80 percent immediately following surgery. They also supported the implementation of an insulin protocol that would minimize the risks of both hyper- and hypoglycemia.17
Howard, Steuber, and Nisly7 conducted a review of the literature to understand the current body of evidence on perioperative BG management in the bariatric surgical environment. Research methods were limited to English language clinical trials and included searching PubMed and MEDLINE from1964 through March 2018. In addition, inclusion criteria required insulin or oral antihyperglycemic medication management, specific to bariatric surgery, and evaluation of clinical outcomes. There were five articles that met the inclusion criteria; two were specific to the immediate postoperative time and three were related to long-term glucose management. Each study used differing protocols and definitions of hyper- and hypoglycemia. It was indicated that patients with a fasting blood glucose level less than 145mg/dL were more likely to achieve complete remission of diabetes, compared to those not managed on an insulin protocol.7
Evidence synthesis. Perioperative hyperglycemia is correlated with surgical complications and reduced mortality.15,16 The use of standardized perioperative insulin protocols has been found to result in better postoperative BG stability than without the use of protocols.6,7,9,15,17 Optimal BG following bariatric surgery is highly predictive of diabetes resolution.6,7 Target BG should be less than 180mg/dL.6,10,16,17 Hypoglycemia did not occur with the use of any glucose protocol.6,7,9
Evidence-based Practice Initiative
Approvals and team recruitment. Institutional Review Board (IRB) approval was not required for this evidence-based initiative. Organizational approvals and any needed professional approvals were identified and completed by the clinical nurse specialist (CNS) and author, who was the acting change agent and facilitator for this initiative. Additional recruited team members consisted of an anesthesiologist and nurses from the bariatric clinic, including a nurse practitioner and perianesthesia/perioperative nurses, which included supervisors, managers, and educators.
Data collection. Preintervention data was collected by the CNS through chart audits beginning January 1, 2020, through June 30, 2021. There was a total of 104 bariatric surgeries and 38 patients with T2D. Preliminary data consisted of BG levels in the perioperative phases of care and surgical unit through 24 hours postoperatively or discharge from the hospital. An incidental finding was higher-than-expected intraoperative dexamethasone dosing. The facility’s ERAS pathway recommends a 4mg dose of dexamethasone for all patients with diabetes. A 10mg dose of dexamethasone will raise BG 20 percent from baseline in patients with and without diabetes. A 4mg dose is sufficient to prevent postoperative nausea and vomiting without hyperglycemic complications.18 Dexamethasone dosing was added to the preliminary data collection, with a reminder to the anesthesiologists to provide no more than 4mg intraoperatively per the ERAS pathway.
Postintervention data was collected by the author through chart audits beginning October 1, 2021, and spanning through July 31, 2022. There was a total of 91 bariatric surgeries and 20 patients with T2D.
Interventions
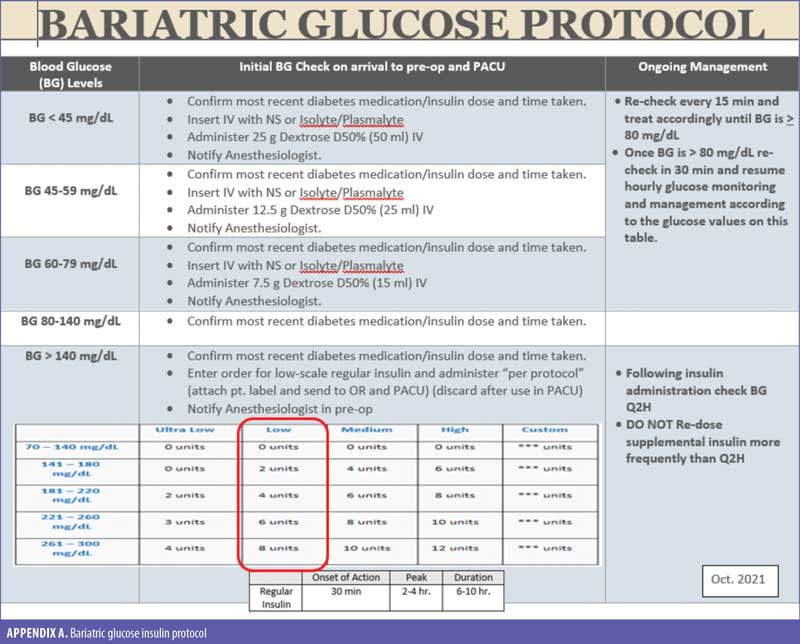
Insulin protocol development. The multidisciplinary team came together and evaluated three insulin protocols from different organizations and the internal insulin scales. An insulin protocol was developed (Appendix A) and shared with the perianesthesia nurses for feedback. Some minor adjustments were made to the protocol, and it was then presented to the anesthesia group, the surgeon group, and the perioperative nursing leadership for approval. Following recommendations discovered in the literature review, the team defined optimal blood glucose management as “all point of care checks less than 180mg/dL after discharge from PACU.” Initially, there were three checkpoints that must have been completed for the protocol to be deemed successful:
- BG checked and protocol used preoperatively
- Intraoperative dexamethasone dosing less than 5mg
- BG checked and protocol used in PACU
However, upon post-initiative evaluation, it was noted that the intraoperative dosing of dexamethasone was not clinically significant (Figure 1) in postoperative glucose stability in this population and was therefore removed as a qualifier for protocol success.
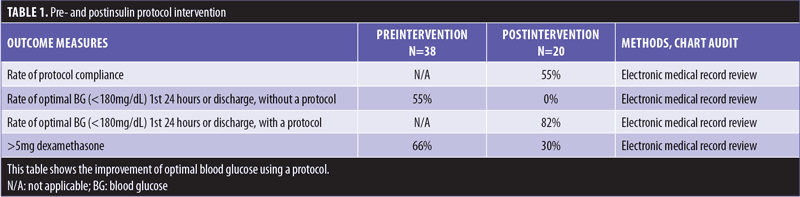
Nursing education development. Nursing education consisted of a PowerPoint presentation that included our hospital’s baseline data (Table 1), along with the evidence review, clarifying why the change was needed at this time. Following this presentation, copies of the glucose protocol (Appendix A) were shared along with instructions on how to order the insulin per protocol. A one-page flyer was created by the author and was posted in visible locations to act as visual reminders (Appendix B). Additionally, the preoperative and PACU supervisors reinforced the evidence-based practice initiative by providing “just in time” reminders to the perianesthesia nurses on the day of surgery.

Barriers to implementation. Barriers to implementation were centered around the COVID-19 pandemic and the resulting nursing staff shortages, coupled with elective surgeries being cancelled due to over 100 percent hospital capacity. The nursing shortage resulted in all nursing leaders being pulled into staffing on other units. There were also noted barriers to implementation due to high nurse turnover and the need to continuously reeducate. Limitations to this evidence-based practice initiative are due to the low volume of bariatric surgeries during the COVID-19 pandemic.
Outcomes
Surprisingly, the intraoperative dexamethasone dosing proved to be clinically insignificant in this population (Figure 1). Utilizing the insulin protocol improved optimal postoperative BG by 49 percent. None of the patients that failed the protocol achieved optimal BG following bariatric surgery (Table 1). By patient report, all patients carbohydrate loaded within four hours prior to surgery, with 35 percent requiring insulin preoperatively. Incidentally, data was collected on patients diagnosed with prediabetes, and while this population was not utilized in this initiative, it was interesting to note that none of these patients were preoperatively hyperglycemic following carbohydrate loading.
Discussion
Even though the entire protocol was only used successfully on 11 patients, the outcomes were promising, with 82 percent of these patients achieving optimal glucose control following bariatric surgery. There were two patients who followed the insulin protocol but failed optimal postoperative BG. One patient who failed despite using the protocol had a hemoglobin A1c (HbA1c) greater than 10 percent. The bariatric program typically requires the patient’s HbA1c to be less than eight percent to qualify for bariatric surgery. It is unknown why the bariatric team deemed the benefit was worth the added risk for this patient when determining suitability for surgery. The second patient that failed despite the use of the protocol only had one point of care BG check greater than 180mg/dL (actual BG: 185mg/dL) and all others were within the goal range. This evidence-based practice initiative will continue and be evaluated following 50 T2D patients successfully using the new insulin protocol.
Conclusion
The global incidence rate of obesity continues to grow. In the US, obesity is rapidly rising, even among youth. Obesity increases the risks of developing many chronic diseases, including diabetes, adding to the national burden of escalating healthcare costs. Bariatric surgery is rapidly becoming a solution to prevent the development of chronic disease, and we should expect this practice will continue to grow in the US. Uncontrolled hyperglycemia during the perioperative environment is known to increase the risks of multiple, postoperative complications, including surgical site infections and delayed resolution of Type 2 diabetes following bariatric surgery. Postoperative BG is best controlled using a perioperative insulin protocol. High reliability organizations should implement standardized protocols whenever possible to improve patient safety and ensure the best surgical outcomes.
References
- Global Burden of Disease collaborative network. Global Buden of Disease Study 2019 (GBD 2019) reference life table. Institute for Health Metrics and evaluation (IHME). 2021. https://ghdx.healthdata.org/record/ihme-data/global-burden-disease-study-2019-gbd-2019-reference-life-table. Accessed 14 Oct 2022.
- Syn NL, Cummings DE, Wang LZ, et al. Association of metabolic-bariatric surgery with long-term survival in adults with and without diabetes: a one-stage meta-analysis of matched cohort and prospective controlled studies with 174,772 participants. Lancet. 2021;397(10287):1830–1841.
- Mechanick JI, Apovian C, Brethaurer S, et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures-2019 update: cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic and Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Endocr Pract. 2019;25(12):1–75.
- Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care. 2016;39(6):861–877.
- American Diabetes Association. 8. Obesity management for the treatment of type 2 diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44(Suppl. 1):S100–S110.
- Zaman JA, Shah N, Leverson GE, et al. The effects of optimal perioperative glucose control on morbidly obese patients undergoing bariatric surgery. Surg Endosc. 2017;31(3):1407–1413.
- Howard ML, Steuber TD, Nisley SA. Glycemic management in the bariatric surgery population: a review of the literature. Pharmacotherapy. 2018;38(6): 663–673.
- American Diabetes Association. 15. Diabetes care in the hospital: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021.44(Suppl. 1): S211–S220.
- Polderman JAW, van Steen SC, Thiel B, et al. Peri-operative management of patients with Type-2 diabetes mellitus undergoing non-cardiac surgery using liraglutide, glucose-insulin-potassium infusion or intravenous insulin bolus regiments: a randomized controlled trial. Anaesthesia. 2018;73(3):332–339.
- Ljungqvist O. Jonathan E. Rhoads lecture 2011: Insulin resistance and enhanced recovery after surgery. J Paren Enteral Nutr. 2012;36(4):389–398.
- Ljunggren S, Hahn RG, Nystrom T. Insulin sensitivity and beta-cell function after carbohydrate oral loading in hip replacement surgery: a double-blind, randomized controlled clinical trial. Clin Nutr. 2014;33(3):392–398.
- Talutis SD, Lee SY, Cheng D, Rosenkranz P, et al. The impact of preoperative carbohydrate loading on patients with type II diabetes in an enhanced recovery after surgery protocol. Am J Surg. 2020;220(4):999–1003.
- Gustafsson UO, Nygren J, Thorell A, et al. Pre-operative carbohydrate loading may be used in Type 2 diabetes patients. Acta Anaesthesiol Scand. 2008;52(7):946–951.
- Robinson KN, Cassady BA, Hegazi RA, Wischmeyer P. Preoperative carbohydrate loading in surgical patients with type 2 diabetes: are concerns supported by data? Clin Nutr ESPEN. 2021;45:1–8.
- Ehrenfeld J, Wanderer JP, Phil M, et al. A perioperative systems design to improve intraoperative glucose monitoring is associated with a reduction in surgical site infections in a diabetic patient population. Anesthesiology. 2017;126(3):431–440.
- Duggan EW, Klopman MA, Berry AJ. The Emory University perioperative algorithm for the management of hyperglycemia and diabetes in non-cardiac surgery patients. Curr Diab Rep. 2016;16(3):34.
- Kheniser G, Kashyap SR. Diabetes management before, during, and after bariatric and metabolic surgery. J Diabetes Complications. 2018;32(9):870–875.
- Joshin GP, Chung F, Vann MS, et al. Society for Ambulatory Anesthesia consensus statement on perioperative blood glucose management in diabetic patients undergoing ambulatory surgery. Anesth Analg. 2010;111(6):1378–1387.
Category: Past Articles, The Viewpoint



