Preoperative Weight Control Strategies Impact Postoperative Outcomes
 by Bridget A. Hannon, MS; Katie N. Robinson, PhD, RD, MPH; Blair Rowitz, MD; Uretz Oliphant, MD; Sharon M. Donovan, PhD, RD; and Margarita Teran-Garcia, MD, PhD, FTOS
by Bridget A. Hannon, MS; Katie N. Robinson, PhD, RD, MPH; Blair Rowitz, MD; Uretz Oliphant, MD; Sharon M. Donovan, PhD, RD; and Margarita Teran-Garcia, MD, PhD, FTOS
Ms. Hannon and Drs. Rowitz, Donovan, and Teran-Garcia are with the Division of Nutritional Sciences, University of Illinois at Urbana-Champaign in Urbana, Illinois. Dr. Robinson is with Scientific and Medical Affairs, Abbott Nutrition, in Columbus, Ohio. Drs. Rowitz and Oliphant are with the Department of Bariatrics, Carle Foundation Hospital in Urbana, Illinois. Dr. Donovan is with the Department of Food Science and Human Nutrition, University of Illinois at Urbana-Champaign in Urbana, Illinois. Dr. Teran-Garcia is with the Department of Human Development and Family Studies, University of Illinois at Urbana-Champaign in Urbana, Illinois.
Funding: This research was supported by the Agriculture and Food Research Initiative of the USDA National Institute of Food and Agriculture as part of the AFRI Childhood Obesity Prevention Challenge (2011-67001-30101) to the University of Illinois. Research was also supported by a generous grant from the Neal Family Research Fund at Carle Foundation Hospital. Ms. Hannon and Dr. Teran-Garcia are supported by the AFRI Competitive Grant no. 2015-68001-23248 to Cooperative Extension and the Department of Human Development and Family Studies at the University of Illinois at Urbana-Champaign.
Disclosures: Katie Robinson is currently an employee of Abbott Nutrition but was not at the time this study was conducted. All other authors have no conflicts of interest relevant to the content of this article.
Abstract: Objective: The goal was to determine associations between preoperative weight control strategies (WCS), weight cycling, and postoperative outcomes following sleeve gastrectomy (SG). Design: This was a longitudinal cohort study with assessments two-weeks preoperatively, day of surgery, and six-weeks and one-year postoperatively. Setting: The study took place at a regional hospital and the University in Illinois. Participants: The study included adults (N=22) with obesity undergoing SG. Measurements: Body composition (body mass index, fat mass [FM], lean body mass [LBM], and body weight [BW], and percent body fat) were assessed at all time points. At one year, postsurgery participants reported the number and type of WCS made prior to surgery. Number of weight loss and regain cycles were also reported. Results: Patients reported an average number of 12.4 (standard deviation: 5.0) preoperative WCS. Higher WCS was associated with body composition changes at one year postsurgery. Each additional WCS was associated with a 0.53 unit decrease in BW (95% confidence interval [CI]: -2.46, -0.35, P=0.01) and a 0.45 unit decrease in FM (95% CI: -1.80, -0.07, P=0.04). Weight cycling was not associated to any outcomes. Conclusion: Patients reporting more WCS had greater loss in both BW and FM one year postoperatively. Frequency of weight cycling was not associated with long-term outcomes. These findings indicate that patients who have had multiple WCS prior to surgery might experience greater weight loss at one year postsurgery than patients who have not attempted extensive means of weight loss, suggesting the patients might have unique behavioral or biological traits that make surgery more effective. Longitudinal research is needed to determine the biological and behavioral reasons for weight regain.
Keywords: Preoperative weight loss, weight cycling, body composition
Bariatric Times. 2020;17(2):12–13.
Bariatric surgery remains the most effective treatment for obesity, a chronic disease that affects 40 percent of adults in the United States.1 However, as with behavioral and pharmaceutical interventions, weight regain is common. Weight regain has been cited to be as high as 50 percent in a cohort of patients 24 months postoperatively.2 However, estimates of the prevalence of weight regain following bariatric surgery are difficult to estimate due to lack of a clear definition of weight regain, and few studies have been published on long-term follow up of patients. The biological and behavioral etiologies behind weight regain are not well understood. Behavioral factors that have been associated with weight regain include postoperative food urges or addictive behaviors.3 A biological reason for weight regain might be compensatory decreases in resting metabolic rate, leading to decreased energy expenditure (EE), which can continue to decrease as the patient loses weight.4,5 Postoperative changes in gut hormone secretion might also contribute to weight regain.6 Much of the research investigating factors associated with weight regain has examined these determinants during the postoperative, not preoperative, period.
Prior to surgery, patients often attempt other behavioral or pharmaceutical weight loss strategies. It might be a prerequisite for surgery that patients demonstrate they are not able to achieve clinically significant weight loss with nonsurgical treatments, which may or may not be physician-supervised. Physician-supervised preoperative weight loss programs have mixed effects on postsurgical outcomes.7 As the number of Americans attempting dietary strategies for weight loss continues to rise,8 it is possible that bariatric patients had made attempts to lose weight prior to consideration of surgery. The phenomenon of weight loss followed by unintentional weight regain is frequently called yo-yo dieting or weight cycling. Weight cycling can lead to decreases in EE and has been associated with increased cardiometabolic risk and sarcopenia.9–11 The prevalence of weight cycling prior to a patient’s consideration of surgical treatment is not well known, nor is the categorization of attempted weight control strategies. The objective of this study was to retrospectively investigate the effects of weight cycling and weight control strategies (WCS) used prior to surgery on postoperative outcomes in patients who underwent bariatric surgery.
Methods
Adults (≥18 years of age) undergoing sleeve gastrectomy (SG) were recruited for the longitudinal Inflammatory Outcomes of Weight Loss Surgery (I-OWLS) cohort study.12 Patients were excluded if they were current smokers or had a pacemaker or implanted defibrillator.
Participants were assessed two weeks prior to surgery (T0), on the day of surgery (T1), six-weeks postoperatively (T2), and one year postoperatively (T3). At each visit, participants underwent a bioelectric impedance analysis (BIA) scan (InBody 230, InBodyUSA, Cerritos, California) to determine body weight (BW), fat mass (FM), lean body mass (LBM), and percent body fat (PBF). Prior to each appointment, participants were asked to fast overnight, avoid alcohol for 24 hours, and avoid exercise for 12 hours to maximize accuracy of InBody assessment. At the one-year postoperative visit, participants were given a list of possible WCSs and asked to report the number and type of WCSs attempted prior to surgery. Additionally, participants were asked to report the number of times they had weight cycled, which was defined as a loss or gain of 10 pounds or more.13
All data were assessed for normality, and nonnormally distributed variables were transformed. Linear regression was used to determine associations between number or WCS or weight cycles and anthropometric measurements at all time points. A P<0.05 was considered statistically significant. SAS 9.4 (SAS Institute, Cary, North Carolina) was used for all analysis.
All study procedures were approved by the Institutional Review Boards of the University of Illinois at Urbana-Champaign and Carle Foundation Hospital. Informed consent was obtained prior to any data collection.
Results
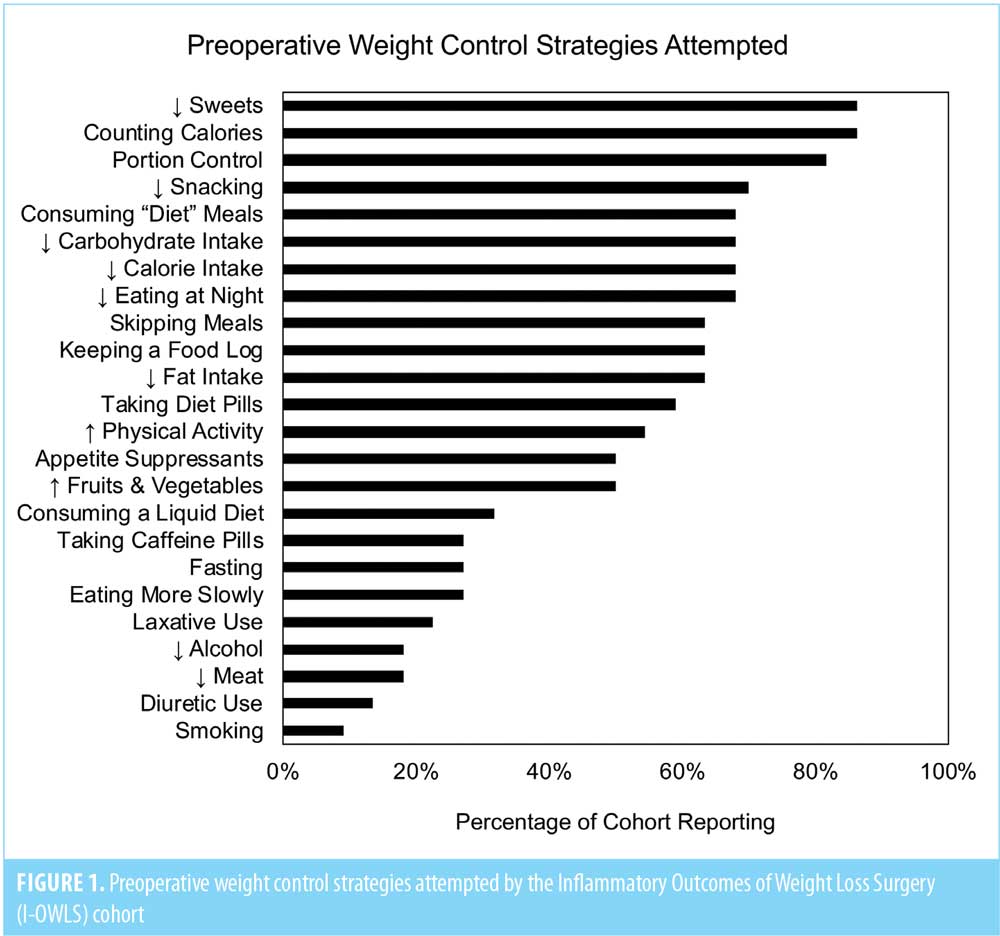
Twenty-two participants (18 female, 4 male) were followed for one year postoperatively. The mean age at T0 was 45.4± standard deviation (SD) 8.6 years. Changes in body composition are shown in Table 1. BMI, BW, FM, LBM, and PBF all significantly decreased between T0 and T3. The mean number of WCS attempted was 12.4±5.0. The mean number of weight cycles reported was 10.3±8.6, which was not significantly different by sex (data not shown). WCSs were significantly higher among women (13.7±4.5) than men (6.5±0.6, P=0.005). The frequencies of each WCS reported are depicted as the percentage of the cohort in Figure 1. The most commonly reported WCSs were decreasing sweets (86%), calorie counting (86%), portion control (82%), and decreasing snacks (70%). Least commonly reported WCSs were smoking (9%), diuretic use (14%), and decreasing meat (18%) or alcohol (18%).
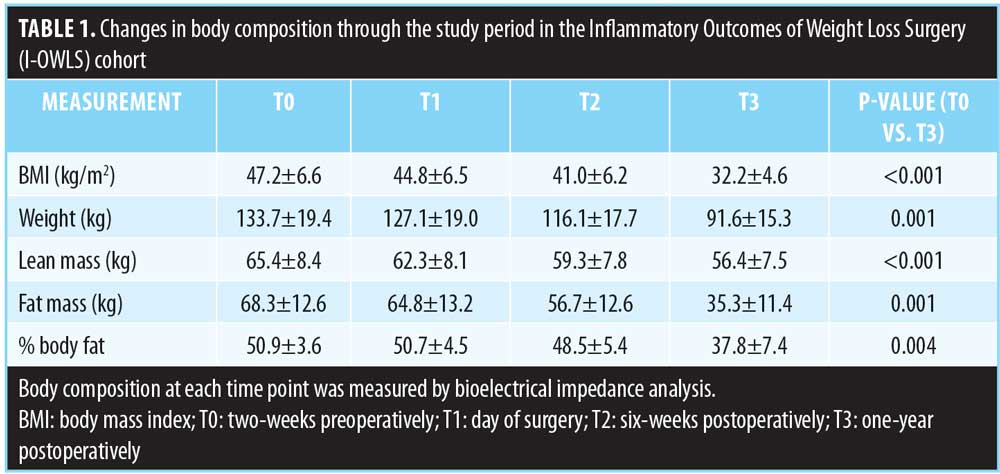
Number of WCSs attempted was significantly associated with losses in BW, FM, and LM one-year postoperatively. Each additional WCS was associated with a 0.53-unit decrease in BW (95% confidence interval [CI]: -2.46, -0.35, P=0.01), a 0.45 unit decrease in FM (95% CI: -1.80, -0.07, P=0.04), or a 0.52-unit decrease in LM (95% CI: -0.84, -0.11, P=0.01). There were no significant associations detected between total WCS and PBF loss. Additionally, the total number of weight cycles did not significantly affect changes in body composition (all P>0.05).
Discussion
These findings highlight the relationship between preoperative weight management behaviors and postoperative outcomes in patients undergoing SG. We examined the role of weight cycling and WCS undergone prior to surgery. Interestingly, frequency of weight cycling, which has been evidenced to impact energy expenditure and ability to lose weight, was not associated with postoperative outcomes. Individuals attempting multiple WCS prior to consideration of surgery experienced greater weight loss and FM loss. These findings suggest that bariatric surgery might be most effective for individuals who have previously attempted multiple different strategies for weight management.
Weight regain after all obesity treatments is common and might be associated with nonadherence to diet or exercise prescriptions, decreases in skeletal muscle mass and energy expenditure, and/or depressive symptoms.14 It is important that the healthcare team is aware of the causes of weight regain and understand preoperative behavioral and biological factors that can be predictive of weight regain. The National Weight Control Registry maintains a database of strategies that have been employed by individuals who have maintained weight loss via surgical and nonsurgical methods. Individuals who do not experience weight regain engage in regular physical activity, track their dietary intake, and regularly self-weigh.15 While this information can inform clinical practice for postoperative patient recommendations, it does not allow researchers to examine predictors of weight regain following surgery.
Previous work has examined patient demographic and metabolic factors that can predict postsurgical weight loss. Preoperative presence of diabetes was repeatedly associated with lower weight loss, as was hypertension, increased age, earlier onset of obesity, depression, and history of psychiatric disorders.16–19 There are few studies available on preoperative behaviors and weight loss outcomes. Subramaniam et al19 reported an association between higher emotional eating behavior and lower weight loss at six months postoperatively; however, these researchers did not state how long before surgery the baseline assessment occurred. Presurgical weight loss greater than 50 pounds was associated with reduced excess body weight loss at one year in a study by Fox et al;17 however, it is not clear how this weight loss was achieved or how long before surgery this loss (and potential regain) occurred. There is a lack of data on individuals prior to consideration of bariatric surgery; thus, researchers must rely on retrospective designs or access to electronic medical records to determine weight cycling history.
Limitations. This study was not without limitations. One limitation is the small sample size of 22. We were unable to substratify and determine which preoperative weight loss strategy (or combination of strategies) was associated with the greatest weight loss at one year. This retrospective design relied on a patient’s memory of WCSs attempted prior to surgery and thus was subject to their ability to remember all attempts. We defined weight cycling as loss and regain of 10 pounds or more, but there are multiple definitions that have been used in the literature, including at least five percent BW, at least 4.5kg, or at least 9kg.20 Thus, the lack of consistent terminology used in the field could be considered a limitation. There have been attempts made by the medical community to standardize terminology for postoperative weight regain, but there is still no consistent definition.21 This study did have several strengths. Body composition was used, rather than mean weight change or BMI alone. Changes in FM and LBM following bariatric surgery impact the individual’s energy expenditure and metabolic ability to lose weight; thus, it is important to characterize how these components change longitudinally. Another strength of this study was the examination of patients undergoing SG, a procedure that is becoming increasingly common.22 Additionally, there is limited published research on preoperative weight loss behaviors in patients undergoing bariatric surgery.
Conclusion
In conclusion, multiple preoperative weight control strategies were associated with increased weight loss following weight loss surgery in our patient sample. Further research is needed to determine the biological and behavioral factors associated with surgical outcomes in helping to identify appropriate surgical candidates and to achieve optimal outcomes.
References
- Hales CM, Fryar CD, Carroll MD, et al. Trends in obesity and severe obesity prevalence in us youth and adults by sex and age, 2007-2008 to 2015-2016. JAMA. 2018;319:1723–1725.
- Magro DO, Geloneze B, Delfini R, et al. Long-term weight regain after gastric bypass: a 5-year prospective study. Obes Surg. 2008;18:648–651.
- Odom J, Zalesin KC, Washington TL, et al. Behavioral predictors of weight regain after bariatric surgery. Obes Surg. 2010;20:349–356.
- Carrasco F, Papapietro K, Csendes A, et al. Changes in resting energy expenditure and body composition after weight loss following Roux-en-Y gastric bypass. Obes Surg. 2007;17:608.
- Maclean PS, Bergouignan A, Cornier M-A, Jackman MR. Biology’s response to dieting: the impetus for weight regain. Am J Physiol Regul Integr Comp Physiol. 2011;301:R581–R600.
- Santo MA, Riccioppo D, Pajecki D, et al. Weight regain after gastric bypass: influence of gut hormones. Obes Surg. 2016;26:919–925.
- Goldenberg L. Weight loss before weight loss surgery: what do we know about dropping those preoperative pounds? Bariatric Times. 2010;7:18–20.
- Goldberg JP, Tanskey LA, Sanders EA, Edge MS. The IFIC Foundation Food and Health Survey 2015: 10-year trends and emerging issues. J Acad Nutr Diet. 117(3):355–357.
- Beavers DP, Beavers KM, Lyles MF, Nicklas BJ. Cardiometabolic risk after weight loss and subsequent weight regain in overweight and obese postmenopausal women. J Gerontol A Biol Sci Med Sci. 2013;68:691–698.
- Bosy-Westphal A, Schautz B, Lagerpusch M, et al. Effect of weight loss and regain on adipose tissue distribution, composition of lean mass and resting energy expenditure in young overweight and obese adults. Int J Obes (Lond). 2013;37:1371–1377.
- Rossi AP, Rubele S, Calugi S, et al. Weight cycling as a risk factor for low muscle mass and strength in a population of males and females with obesity. Obesity. 2019;27:1068–1075.
- Robinson KN, Rowitz B, Oliphant UJ, et al. Larger omental adipocytes correlate with greater Fetuin-A reduction following sleeve gastrectomy. BMC Obesity. 2019;6:15.
- Stevens VL, Jacobs EJ, Sun J, et al. Weight cycling and mortality in a large prospective US study. Am J Epidemiol. 2012;175:785–792.
- Still C. Weight regain following obesity treatment is not only common, it’s expected August 1, 2019. https://bariatrictimes.com/chris-still-editorial-august-2019/. Accessed January 21, 2020.
- Thomas JG, Bond DS, Phelan S, et al. Weight-loss maintenance for 10 years in the National Weight Control Registry. Am J Prev Med. 2014;46:17–23.
- Campos GM, Rabl C, Mulligan K, et al. Factors associated with weight loss after gastric bypass. Arch Surg. 2008;143:877–883; discussion 884.
- Fox B, Chen E, Suzo A, et al. Dietary and psych predictors of weight loss after gastric bypass. J Surg Res. 2015;197:283–290.
- Sillen L, Andersson E. Patient factors predicting weight loss after Roux-en-Y gastric bypass. J Obes. 2017;2017:3278751.
- Subramaniam K, Low WY, Lau PC, et al. Eating behaviour predicts weight loss six months after bariatric surgery: a longitudinal study. Nutrients. 2018;10.
- Mehta T, Smith DL Jr., Muhammad J, Casazza K. Impact of weight cycling on risk of morbidity and mortality. Obes Rev. 2014;15:870–881.
- King WC, Hinerman AS, Belle SH, et al. Comparison of the performance of common measures of weight regain after bariatric surgery for association with clinical outcomes. JAMA. 2018;320:1560–1569.
- English WJ, DeMaria EJ, Brethauer SA, et al. American Society for Metabolic and Bariatric Surgery estimation of metabolic and bariatric procedures performed in the United States in 2016. Surg Obes Relat Dis. 2018;14:259–263.
Category: Brief Report, Past Articles



