Nasogastric Tube Perforation Masquerading as a Delayed Gastric Sleeve Leak
 by David J. Morrell, MD; Samantha R. Witte, MD; Gustavo Bello, MD, FACS; Ann M. Rogers, MD, FACS, FASMBS; and Eric M. Pauli, MD, FACS, FASGE
by David J. Morrell, MD; Samantha R. Witte, MD; Gustavo Bello, MD, FACS; Ann M. Rogers, MD, FACS, FASMBS; and Eric M. Pauli, MD, FACS, FASGE
Drs. Morrell, Rogers, and Pauli are with the Department of Surgery, Penn State Health Milton S. Hershey Medical Center in Hershey, Pennsylvania. Dr. Witte is with General and Bariatric Surgery at Bryn Mawr Hospital, Main Line Health in Bryn Mawr, Pennsylvania. Dr. Bello is with the Department of Surgery, Greater Baltimore Medical Center in Towson, Maryland.
Funding: No funding was provided.
Disclosures: Eric M. Pauli is a consultant, has received research grants, and/or is a speaker for Boston Scientific, Cook, Bard, Medtronic, Actuated Medical, Springer, and UpToDate. All other authors have no conflicts of interest relevant to the content of this article.
Abstract: Delayed gastric sleeve leak is an uncommon complication of sleeve gastrectomy (SG). Here, we present the case of a 39-year-old female patient with history of laparoscopic SG who presented with radiographic evidence of an apparent delayed gastric sleeve leak one year postoperatively. She had undergone a total thyroidectomy one month prior to presentation and developed new abdominal pain and fever. She was found to have a collection adjacent to the sleeve staple line on cross-sectional imaging. After percutaneous drainage of the intrabdominal collection and initiation of total parenteral nutrition (TPN), she was referred for endoscopic evaluation and management. Although not documented in the operative or anesthesia records, the patient’s spouse recalled that an intra-operative placed nasogastric tube (NGT) was discussed before her thyroidectomy due to history of gastroesophageal reflux after SG. Upper endoscopy demonstrated a defect adjacent to the staple line midway between the angle of His and the lesser curve, directly in line with the esophageal lumen, supporting the presumed mechanism of perforation from placement of a NGT during her thyroidectomy. The perforation was successfully managed with multimodal endoscopic therapy, including an over-the-scope clip. She was discharged on a diet, her TPN was stopped, and her extraluminal drain was removed one week following endoscopy. Seven months after the procedure she was tolerating a regular diet and had no further complications. NGT placement in patients with a history of SG can result in perforation. Endoscopic therapy has the potential to manage such perforations utilizing the surgical principles of source control, drainage, and closure.
Keywords: Sleeve gastrectomy, endoscopy, delayed sleeve leak, perforation, over-the-scope clip
Bariatric Times. 2020;17(2):9–11.
Leak following sleeve gastrectomy (SG) is one of the most feared complications of a relatively safe bariatric procedure. The incidence of SG leak has been reported to be approximately two percent in several systematic reviews, with the majority of leaks occurring in the proximal staple line.1–3 Delayed presentations of SG leaks are even more uncommon. In one multicenter study with 2,834 patients and a gastric leak rate of 1.5 percent, only seven percent of those leaks presented more than 14 days postoperatively.4 In that same study, 75 percent of leaks were located near the gastroesophageal junction (GEJ).
In comparison, gastric perforation from nasogastric tube (NGT) placement is an uncommon occurrence. The rare nature of this complication is evident in that prior literature consists only of case reports.5–7 Antecedent gastric surgery has been suggested as a risk factor for NGT-related perforation.8 In patients who have undergone bariatric surgery, there is often hesitation to blindly place NGTs given a perceived risk of staple line perforation.9,10
The American Society for Metabolic and Bariatric Surgery (ASMBS) includes endoscopic management as an appropriate option for management of SG leaks.11 A variety of endoscopic therapeutic modalities have been used to date to manage these leaks, including endoluminal stents, endoscopic clips, fibrin sealant, endoluminal suturing devices, endoluminal vacuum therapy, and over-the-scope clips (OTSC).12–16 Here, we present a patient with a NGT perforation of their SG masquerading as a delayed presentation SG leak, which was managed using comprehensive endoscopic therapy.
Case Presentation
A 39-year-old female patient with a history of uncomplicated laparoscopic SG for clinically severe obesity was referred to our facility 16 months after her surgical procedure for management of a presumed delayed SG leak. Her postoperative recovery had been uncomplicated, and 15 months after the SG (1 month prior to referral to our facility), she had undergone a total thyroidectomy for nontoxic multinodular goiter. There were no intraoperative complications noted; however, the patient complained of abdominal pain and fever postoperatively. After a computed tomography (CT) scan of the abdomen and pelvis was obtained and interpreted as normal, she was discharged home.
She presented again to the referring facility one month later with ongoing abdominal pain, fevers, and rigors, which prompted a repeat CT of the abdomen and pelvis. The CT demonstrated a complex collection containing contrast and air adjacent to the gastric body, which was felt to be consistent with a delayed presentation SG leak. Successful percutaneous drainage of this collection was performed, and a peripherally inserted central catheter (PICC) was placed to initiate total parenteral nutrition (TPN). She was subsequently discharged with a referral to our facility for outpatient endoscopic management of this leak.
After review of the operative notes and anesthesia records from the thyroidectomy, we could identify no clear reason why the surgery would suddenly unmask an otherwise asymptomatic leak. We hypothesized that perhaps bag–mask ventilation had resulted in the issue. In discussing this with the patient before endoscopy, her husband spontaneously offered his recollection of having a discussion with the anesthesiologist that a NGT would be used during the thyroidectomy because of the patient’s complaints of severe gastroesophageal reflux symptoms following her SG.
Under general endotracheal anesthesia, a diagnostic esophagogastroduodenoscopy was performed. The SG had a normal overall configuration without evidence of twist or narrowing, and the pylorus was widely patent. We identified a defect adjacent to (but not through) the staple line midway between the angle of His and the lesser curve, in direct alignment with the esophageal lumen. There was adjacent erythematous mucosa (Figure 1). With aspiration, purulent material flowed through the defect into the lumen and with insufflation, the patient’s extraluminal drain was noted to be filling with air. The diagnostic gastroscope was then exchanged for an ultraslim transnasal gastroscope, which was advanced transluminally into the leak cavity (Figure 2) to permit washout, debridement, and evaluation of the percutaneous drain position.
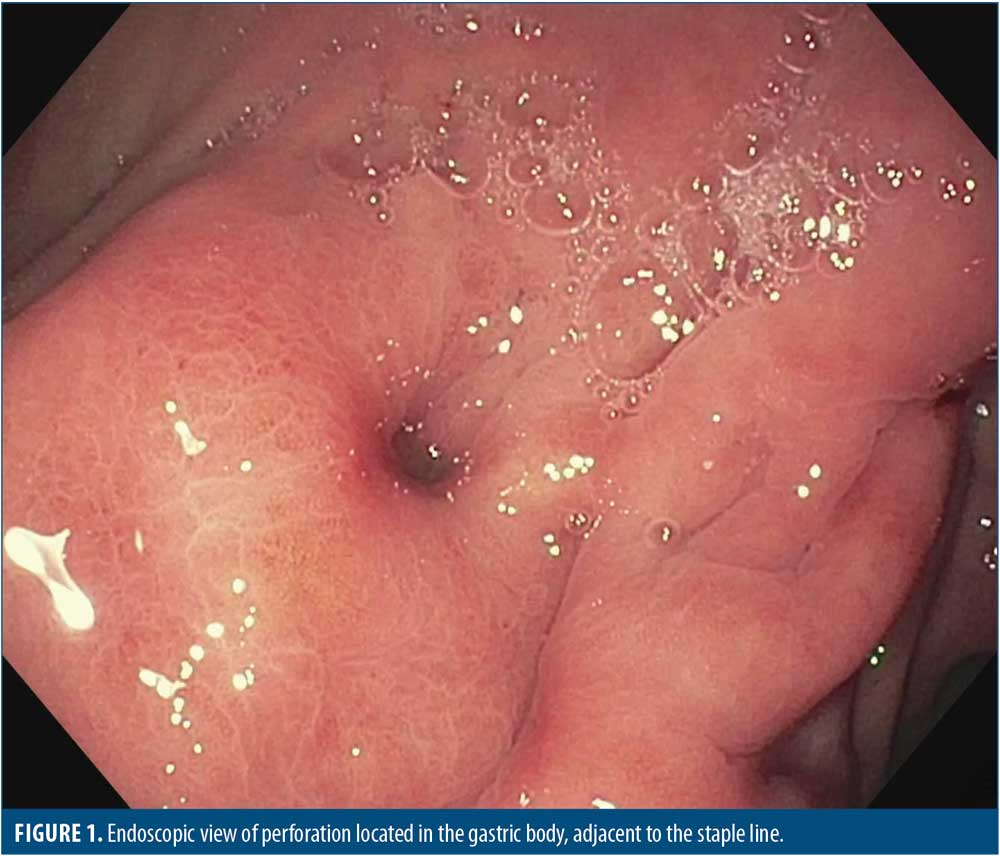
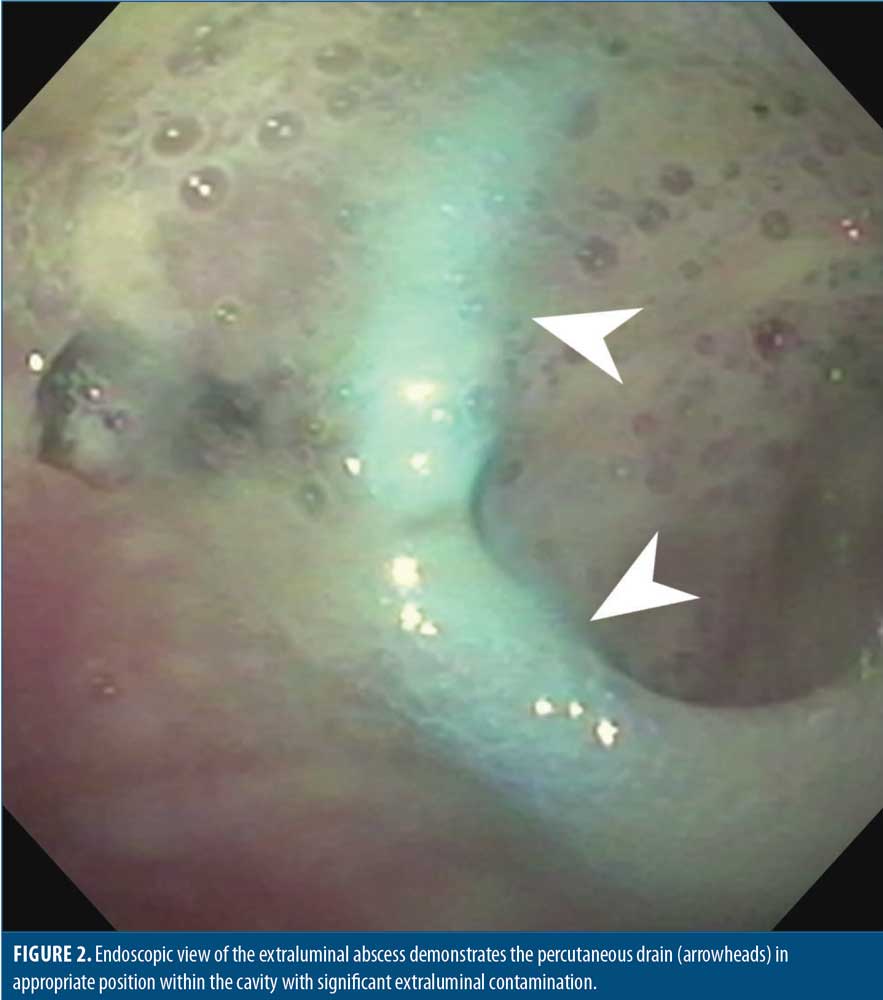
The pathway from the GEJ to the defect was noted to be a straight line both endoscopically and radiographically (Figure 3). The defect location was several centimeters away from the anticipated acute sleeve leak location high on the apex of the staple line. Given these findings, we believed this defect represented a perforation from NGT insertion the previous month rather than a delayed presentation of a postoperative SG leak.
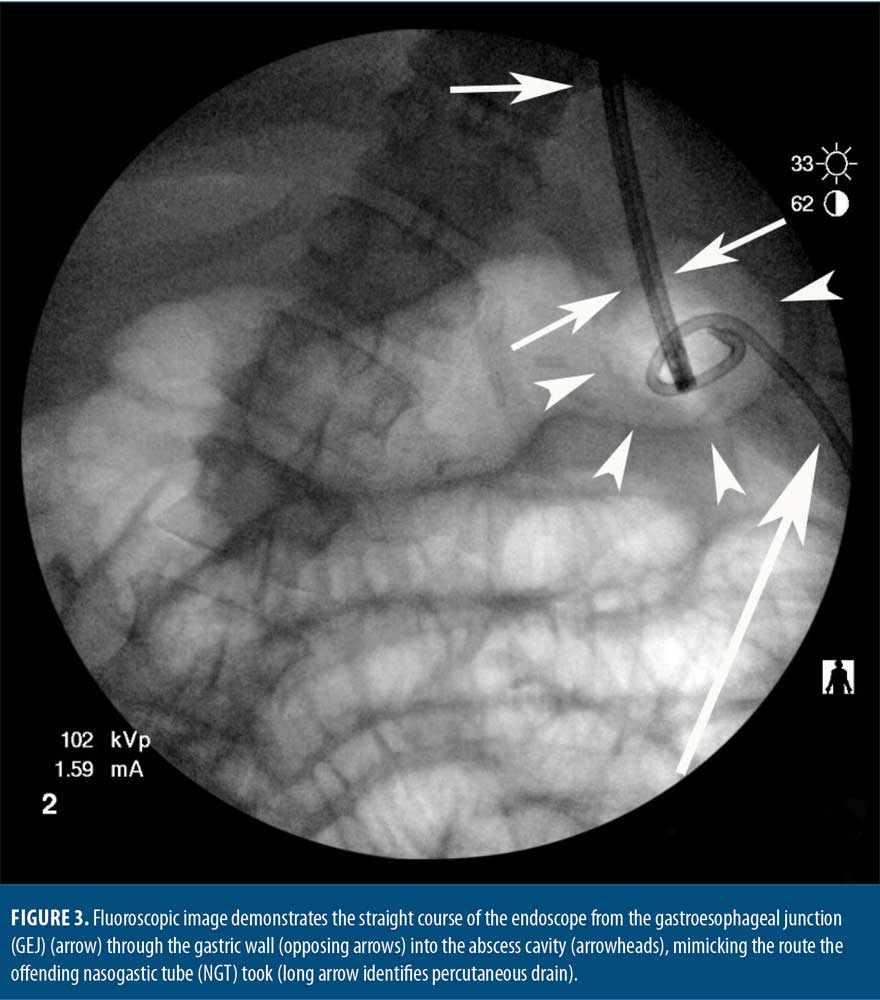
Transluminal endoscopy demonstrated the previously placed percutaneous drain was well-positioned and was flushed to ensure patency. It was situated in the dependent portion of the abscess cavity and was remote from the gastric body to prevent erosion or inadvertent drain capture during endoscopic closure of the gastric defect. The extraluminal cavity was then thoroughly irrigated with 500mL of warm saline to clear all remaining debris. No solid food was encountered. After irrigation, there was no foreign material or debris remaining.
Because this was a nonacute defect, the edges of the gastric perforation were circumferentially ablated using an argon plasma coagulation to allow for subsequent scar formation and to eliminate any epithelium from the tract through the gastric wall. An over-the-scope clip (12/6 GC OTSC, Ovesco Endoscopy AG, Germany) was advanced to the perforation mounted on the end of a therapeutic gastroscope. An anchor grasper was used in combination with aggressive suctioning to pull the defect edges up into the OTSC cap prior to firing the clip.
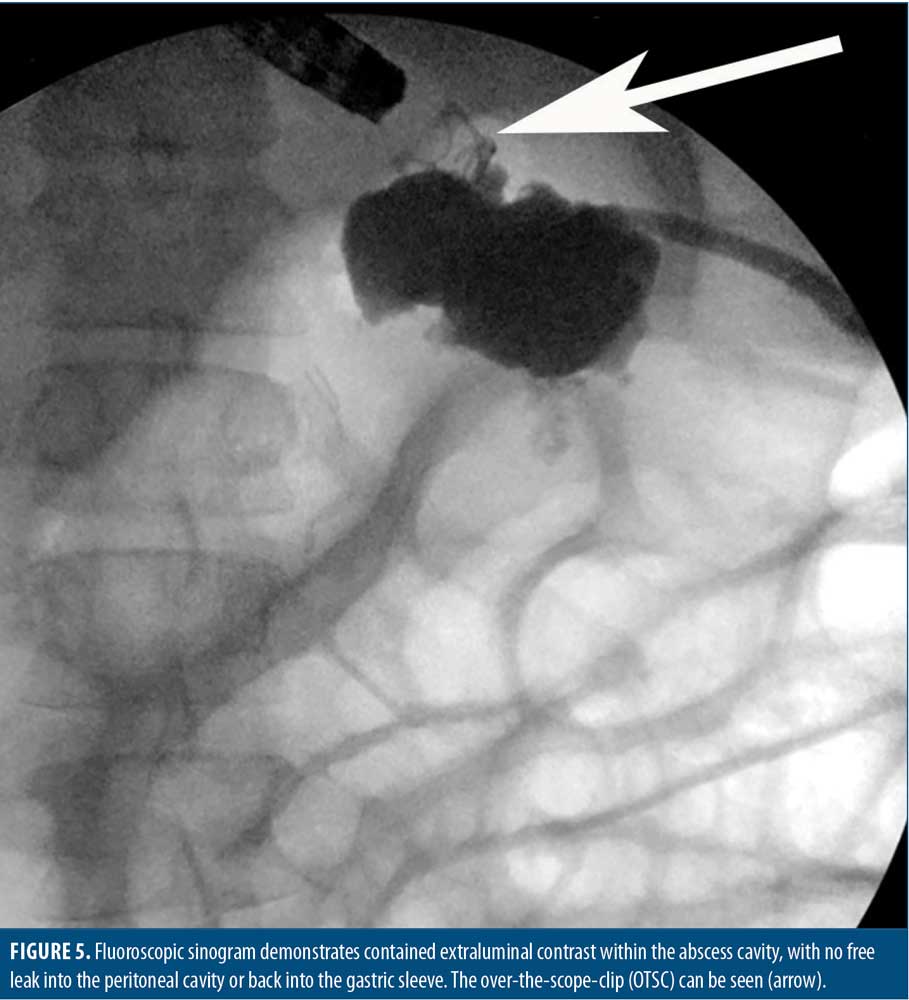
Clip positioning was confirmed endoscopically (Figure 4). Immediately, there was no further evidence of luminal communication with the drain during aggressive carbon dioxide insufflation. A sinogram was performed, which demonstrated extraluminal contrast within a contained abscess cavity (Figure 5). There was no free communication with the peritoneal cavity or the gastric sleeve. The patient tolerated the procedure well, and there were no procedural complications.
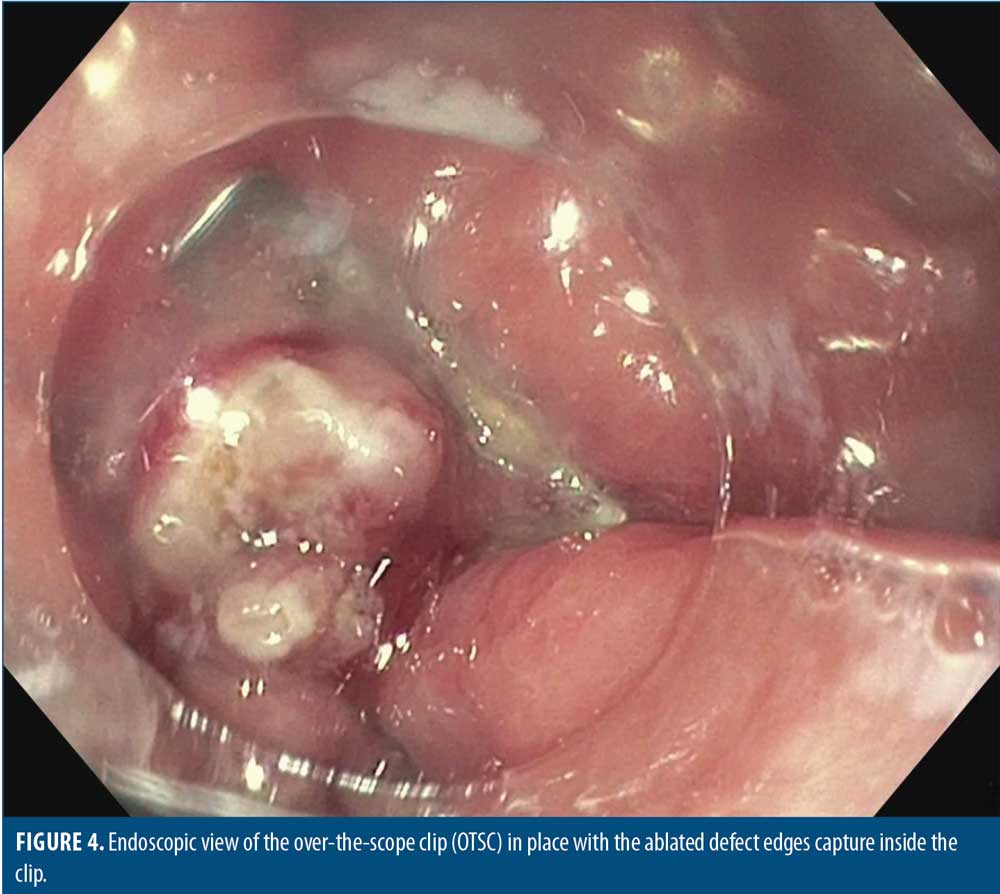
The patient was started on a clear liquid diet and was observed overnight. She was discharged the next day on a full liquid diet with dietary advancement instructions. Her PICC was removed, and her TPN was discontinued. Antibiotics were discontinued. At one week after discharge, the extraluminal drain was removed due to lack of output. She quickly advanced back to a normal post-SG diet. At her seven-month follow-up visit following the endoscopic closure procedure, she remained complication-free.
Discussion
Concern regarding the potential for perforation from gastric instrumentation in the acute postoperative period following SG might cause many surgeons to be hesitant to blindly place NGTs. Although blind placement of NGTs in an animal model was recently demonstrated to not result in perforation, this case would suggest that the risk still remains even in patients with a remote history of SG.10
The initial clinical picture of this patient was indistinguishable from a delayed presentation of a gastric sleeve leak. The key differentiating factor was the location of the gastric defect. Sleeve leaks most commonly occur at the apex of the gastric staple line, near the GEJ—a location that is anatomically unlikely to perforate during NGT placement. The perforation identified endoscopically was directly in line with the GEJ and was located slightly anterior to the staple line itself—a location directly in the path of an advancing NGT. The management of gastrointestinal defects resulting from these two etiologies is identical and consists of source control, which can be accomplished through operative intervention, percutaneous drainage, and/or endoscopic management as was done in this case.
Although gastric decompression via NGT or orogastric tubes is a common practice during procedures conducted under general anesthesia, there is limited literature on the subject. In a systematic review by Long et al,17 the authors recommended against elective NGT placement in the absence of aspiration risk factors or a need for surgical decompression. Under these criteria, our patient would not have required gastric decompression in the perioperative period and therefore would have avoided the inciting event for her gastric perforation. We recommend awareness of the risk of NGT perforation in patients who have had bariatric or other gastric surgeries, communication between surgeon and anesthesia provider about the need for (or lack thereof) an NGT, care during tube placement, and vigilance in the postprocedural period for the sequelae of perforation.
The success of endoscopic management of full-thickness GI defects has been well established by multiple case series. A recent systematic review reported successful endoscopic management of 85 percent for 351 patients treated for perforation and 66 percent for patients treated for anastomotic dehiscence.18 These numbers represent a large proportion of patients that successfully avoided the significant morbidity caused by repeat surgical intervention.
We have recently reported our experience with endoscopic management of nonacute, full-thickness gastrointestinal (GI) defects utilizing multimodal endoscopic therapy and OTSC with a high procedural success rate.19 Our treatment algorithm focuses on implementing surgical principles through an endoscopic intervention and as such focuses on treating underlying infection, optimizing nutrition, removing foreign bodies, de-epithelializing/ablating mucosa, relieving downstream obstructions, and collecting GI effluent.20,21 Only after these issues are addressed do we contemplate endoscopic defect closure. We believe these surgical principles to be critical to successful endoscopic closure of GI defects.20,21
Conclusion
This case demonstrates that the risk of NGT perforation persists even in patients with remote history of SG. These perforations could masquerade as delayed gastric sleeve leaks. This case also demonstrates the successful management of a gastric sleeve perforation using endoscopic washout, closure, and continued extraluminal drainage.
References
- Aurora AR, Khaitan L, Saber AA. Sleeve gastrectomy and the risk of leak: a systematic analysis of 4,888 patients. Surg Endosc. 2012;26(6):1509–1515.
- Gagner M, Buchwald JN. Comparison of laparoscopic sleeve gastrectomy leak rates in four staple-line reinforcement options: a systematic review. Surg Obes Relat Dis. 2014;10(4):713–723.
- Gagner M, Kemmeter P. Comparison of laparoscopic sleeve gastrectomy leak rates in five staple-line reinforcement options: a systematic review. Surg Endosc. 2020;34(1):396–407.
- Sakran N, Goitein D, Raziel A, et al. Gastric leaks after sleeve gastrectomy: a multicenter experience with 2,834 patients. Surg Endosc. 2013;27(1):240–245.
- Janicki A, van Ginckel C, Cohn J. Gastric perforation following nasogastric intubation in an elderly male. R I Med J (2013). 2015;98(9):45–46.
- Daliya P, White TJ, Makhdoomi KR. Gastric perforation in an adult male following nasogastric intubation. Ann R Coll Surg Engl. 2012;94(7):e210–212.
- Ghahremani GG, Turner MA, Port RB. Iatrogenic intubation injuries of the upper gastrointestinal tract in adults. Gastrointest Radiol. 1980;5(1):1–10.
- Hodin RA, Bordeianou L. Inpatient placement and management of nasogastric and nasoenteric tubes in adults, in UpToDate, T. Post, Editor., UpToDate Inc.: Waltham, MA.
- Carrasquilla C, Weiss M, Gianos J. Safe intestinal decompression in fresh postoperative gastric bypass. Obes Surg. 2006;16(9):1256–1260.
- Fabian T, Robinson T, Naile L, et al. Blind nasogastric tube advancement following sleeve gastrectomy: an animal model. Surg Endosc. 2020;34(1):257–260.
- Kim J, Azagury D, Eisenberg D, et al. ASMBS position statement on prevention, detection, and treatment of gastrointestinal leak after gastric bypass and sleeve gastrectomy, including the roles of imaging, surgical exploration, and nonoperative management. Surg Obes Relat Dis. 2015;11(4):739–748.
- Juza RM, Raluck RS, Pauli EM, et al. Gastric sleeve leak: a single institution’s experience with early combined laparoendoscopic management. Surg Obes Relat Dis. 2015;11(1):60–64.
- Southwell T, Lim TH, Ogra R. Endoscopic therapy for treatment of staple line leaks post-laparoscopic sleeve gastrectomy (LSG): experience from a large bariatric surgery centre in New Zealand. Obes Surg. 2016;26(6):1155–1162.
- Moon RC, Shah N, Teixeira AF, Jawad MA. Management of staple line leaks following sleeve gastrectomy. Surg Obes Relat Dis. 2015;11(1):54–59.
- Winder JS, Pauli EM. Comprehensive management of full-thickness luminal defects: The next frontier of gastrointestinal endoscopy. World J Gastrointest Endosc. 2015;7(8):758–768.
- Alazmi W, Al-Sabah S, Ali DA, Almazeedi S. Treating sleeve gastrectomy leak with endoscopic stenting: the Kuwaiti experience and review of recent literature. Surg Endosc. 2014;28(12):3425–3428.
- Long M, Machan M, Tollinche L. Intraoperative gastric tube intubation: a summary of case studies and review of the literature. Open J Anesthesiol. 2017;7(3):43–62.
- Kobara H, Mori H, Nishiyama N, et al. Over-the-scope clip system: a review of 1517 cases over 9 years. J Gastroenterol Hepatol. 2019;34(1):22–30.
- Morrell DJ, Winder JS, Johri A, et al. Over-the-scope clip management of non-acute, full-thickness gastrointestinal defects. Surg Endosc. 2019 Jul 26. [Epub ahead of print]
- Witte SR, Pauli EM. Management of gastrointestinal tract defects. Ann Laparosc Endosc Surg. 2019;4.
- Winder JS, Pauli EM. Novel endoscopic modalities for closure of perforations, leaks and fistula in the gastrointestinal tract. Tech Gastrointest Endosc. 2019;21:109–114.
Category: Case Report, Past Articles



