Protein Intake after Bariatric Surgery: A Review
 by Silvia Leite Faria, PhD; Mariane de Almeida Cardeal, MSci; and Orlando Pereira Faria, MD
by Silvia Leite Faria, PhD; Mariane de Almeida Cardeal, MSci; and Orlando Pereira Faria, MD
Drs. Leite Faria, Pereira Faria, and de Almeida Cardeal are with Gastrocirurgia de Brasília in Asa Sul, Brasília.
FUNDING: No funding was provided.
DISCLOSURES: Silvia Leite Faria is a consultant for Bariatric Advantage. The other authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Bariatric surgery is a complex procedure that involves physiological changes that might eventually affect food tolerances and preferences. Positive qualitative and quantitative weight loss, long-term weight maintenance, and prevention of nutritional deficiencies are the principal targets. For this, an adequate protein intake is crucial.
Objective. The objective is to review the available information regarding protein intake after bariatric surgery.
Methods. Selected content available in scientific literature was reviewed to summarize data on protein consumption related to bariatric surgery and the maintenance of muscle mass, the status of serum proteins, prevention of possible complications, and the need for supplementation.
Results. Post-bariatric surgery patients can reach a loss of 25 percent of their preoperative muscle mass by the end of the first year, mainly during the first trimester. Protein intake of at least 60g/day or 1.1g/kg ideal body weight/day leads to decreased muscle loss. A recent long-term observational study showed better postoperative weight maintenance after 10 years of bariatric surgery among those who preferred protein intake to lipids and carbohydrates. However, only about 40 percent of this population can achieve adequate consumption levels. Low consumption of protein during the weight loss period might lead to the appearance of sarcopenia. Thus, supplements can help, assisting in a better weight loss, while preventing muscle mass loss and weight regain.
Conclusion. The consumption of protein after bariatric surgery seems to avoid excessive loss of muscle mass and helps in long-term weight maintenance, which can be aided by the use of supplements. Long-term, controlled intervention studies with appropriate methodology are necessary to establish a more reliable protein recommendation for this population.
KEYWORDS: Protein, bariatric surgery, weight loss, muscle loss, supplements
Bariatric Times. 2020;17(7):12–14
Bariatric surgery is currently the most effective treatment for clinically severe obesity, ensuring sustained long-term weight loss. In 2018, the IFSO Global Registry reports registered almost 400,000 surgeries were performed worldwide, with sleeve gastrectomy (SG) being most frequently performed, followed by Roux-en-Y gastric bypass (RYGB) surgery.1
SG is a metabolic procedure with gastric restriction and some metabolic effects, including higher production of incretins. RYGB is also a metabolic procedure that combines gastric restriction with intestinal deviation of the proximal duodenum and jejunum.2 In this way, both procedures limit food intake, which is what appears to increase the risk of protein deficiency among patients following surgery, since the macronutrient absorption does not seem to be significantly altered even after the deviation made in RYGB.
The biliopancreatic diversion (BPD) and its duodenal switch (BPD/DS) variant are truly malabsorptive procedures, with both consisting of a distal horizontal gastrectomy that leaves an average of 200mL of upper stomach. This remnant stomach is anastomosed to the distal 250cm of small intestine (alimentary limb). The excluded small intestine (carrying bile and pancreatic secretion), called the biliopancreatic limb, is connected to the small bowel 50cm proximal to the ileocecal valve. The 50cm-long common limb is the only segment in which digestive secretions and nutrients mix, causing an expressive malabsorption, especially for fat and protein.3 Due to this complex intestinal rearrangement, these procedures can lead patients to a higher malnourishment status and, thus, are not commonly used procedures, accounting for less than one percent of all procedures.
Because bariatric surgery is a complex procedure resulting in physiological changes, it can affect patient food tolerances and preferences. Energy restriction is easily reached initially due to surgical changes necessary to achieve significant weight loss. However, bariatric surgery increases the risk for nutritional complications, such as protein deficiency. The main causes for protein deficiency are related to the patient’s inability to absorb adequate amounts of protein from ingested food.
During the weight loss process, if a patient does not consume the adequate amount of protein, sarcopenia can arise. Sarcopenia is a syndrome characterized by progressive and generalized loss of skeletal muscle mass and strength. In patients who undergo bariatric surgery, a 25-percent loss of preoperative muscle mass at one-year post-surgery is currently considered acceptable.4,5 An observational study estimated a progressive reduction of prealbumin over the same period, with about 40 percent of the bariatric surgery population appearing to show mild protein deficiency at one-year post-surgery.6 In addition, protein-calorie malnutrition has been linked to continued post-surgery weight loss for prolonged periods of time, extending beyond two years following surgery. In contrast, the typical pattern of weight loss after malabsorptive procedures is weight loss up to 18 months postoperatively, with weight stabilization or even a small weight regain after this period.7 The results of the more recent studies advocate cautious monitoring of protein intake in patients following bariatric surgery.⁶
We examined the available scientific literature regarding protein consumption and degree of muscle mass loss in patients who have undergone bariatric surgery in an effort to determine the optimal amount of protein post-surgery patients should ingest to reduce risk of muscle mass loss.
Materials and Methods
Information sources. Cochrane, EMBASE, LILACS, PubMed, Scopus, and Web of Science databases were used for our literature search. Articles published between 2004 and 2019 were included. The following words or phrases were used in the search: protein, bariatric surgery, protein and bariatric surgery, protein deficiency, amino acids, protein levels, protein supplements and dietary protein recommendations, and body composition. Selected content was reviewed to summarize data on protein consumption recommendations related to bariatric surgery and the maintenance of muscle mass, prevention of possible complications, the status of serum proteins, and the need for supplementation.
Eligibility criteria. Inclusion criteria. The review included experimental and observational studies of patients after bariatric surgery, mainly RYGB and SG. Studies that measured protein intake at any postoperative moment were considered. Studies evaluating body composition measured by electrical bioimpedance (BIA) and dual-energy X-ray absorptiometry (DXA) and/or serum albumin and prealbumin levels to observe protein deficiency were also considered. Publication in the English language and within the years stated above were also required, with no restrictions as to the publication status.
Exclusion criteria. Studies that included participants under 18 years of age or over 60 years of age were excluded. Letters, conference proceedings, summaries, book chapters, and editorials were also excluded.
Study selection. The article selection was completed in two phases. In Phase 1, two reviewers (MAC and SLF) independently reviewed the titles and abstracts of all electronic database citations. Articles that did not appear to meet the inclusion criteria were discarded. In Phase 2, the same reviewers independently used the inclusion criteria on the full text of the articles. Any disagreement in the first or second phase was resolved by the other author (OPF).
Data collection process. One author (MAC) extracted data from the selected studies. A separate author (SLF) cross-checked all of the information. Any disagreements between the two authors were discussed and resolved by the third author (OPF), based upon the criteria established.
Data items. The following information was recorded from the selected studies: authors’ names, publication year, country, study purpose, sample size, surgery type, mean age, study design, follow-up period, outcomes of interest for the review, use of protein supplementation, whether the patients presented significant loss of muscle mass or decrease of serum albumin/prealbumin, and presence of clinical signs and symptoms related to a low-protein intake.
Results and Discussion
Of 43 retrieved articles, 34 were selected for analysis, including one that presented a guideline, 12 review articles, 15 observational studies (6 were transversal, 8 prospective and one was a case-controlled study), 4 intervention studies (one of which was randomized, controlled and double-blind, 2 were open-label, not controlled, nor randomized and one was randomized, controlled and open-label). Twenty-eight of the studies were related directly to patients who underwent bariatric surgery. Articles that did not address topics related to bariatric surgery, protein intake, and patients with overweight/obesity were excluded. The selected articles examined the general population and patients who underwent bariatric surgery in such a way that the general population provided data that helped to explain protein-related benefits in the postoperational phase of bariatric surgery.
Diagnosis of protein deficiency. Body composition. In conjunction with other factors, the evaluation of body composition should be considered when assessing protein needs. One of the objectives of prioritizing the protein intake for bariatric surgery patients is to ensure weight loss with the maximum preservation of muscle mass, especially in the first year after surgery. The preservation of lean mass is desirable since it is related to a better resting metabolic rate,8,9 which in turn is associated with better long-term maintenance of weight loss and with better treatment outcomes of weight regain.10,11 Although a 25-percent loss of lean mass in relation to total weight loss seems acceptable in clinical practice, there is still no consensus as to just how much loss of this tissue would be acceptable after bariatric procedures. A systematic review found a loss of lean mass ranging from 10.5 to 27.7 percent after RYGB and SG,12 while Chaston et al,4 in their review article, reported an average loss of 31 percent for RYGB patients. Aron-Wisnewsky et al6 studied patients who had undergone RYGB or SG, using DXA, and observed similar characterstics of muscle mass loss between the two procedures in the first postoperative year. Interestingly, Moize et al,13 comparing the same populations and also using DXA as a measurement method, observed that the loss of muscle mass was significantly more intense in patients who had SG, especially at the end of 12 months, postoperatively.13 Such a lack of consensus might be due to small patient numbers or methodology differences in the verification of body composition. However, a recent systematic review concluded that the intestinal deviation characteristic of RYGB did not appear to exert any major influence on protein intake, digestion, or absorption. The authors of the review reported a decrease in the absorption coefficient of less than 5.0 percent compared to the preoperative period.3 Thus, the risk of protein malnutrition, where the loss of muscle mass is considered an evaluative criterion, could be due to low intake or intolerance to sources of protein (e.g., red meat) by the patients, as demonstrated in previous studies.14-16
A recent prospective study comparing RYGB, SG, and adjustable gastric band over five years analyzed body composition with whole-body magnetic resonance imaging (MRI). The investigators reported that patients lost 16 to 23 percent of fat-free mass (FFM) in the first year postoperatively and that patients who underwent RYGB had a greater loss of FFM (35%) than those who underwent SG (27%) or AGB (13%). During the first and second years postoperatively, only patients who underwent AGB had the tendency to maintain their lean body mass. After the second year, the muscle mass loss was attributed to the aging process.17
Cole et al18 analyzed patients who underwent RYGB, reporting that they lost 16 pecent of FFM in the first year postoperatively, and, in Years 2 to 9 postoperatively, there was a decrease of 4kg of FFM and an increase of 8kg of fat mass. There was a correlation between FFM loss and weight regain.
Consensus among the studies appears to be that the loss of lean mass is more intense and significant the first six months following surgery and then stabilizes or mildly decreases until the end of the first year.6,15,19 Adequate protein consumption appears to mitigate this situation.12,20,21
Clinical nutritional evaluation. Allied to the anthropometric evaluation (body composition), the clinical signs and biochemical evaluation are extremely relevant to detect protein depletion and determine how much its intake should be increased. The clinical signs, such as hair loss, poor wound healing, infections after skin repair surgeries, muscle weakness, and fatigue, are characteristics of inadequate protein intake.21–23
Biochemical assessment. Traditionally, protein malnutrition is defined as a manifestation of serum albumin levels below 3.5mg/dL. However, in bariatric surgery patients, the prevalence of hypoalbuminemia does not seem high, becoming more prevalent postoperatively after the first six months. Even so, in some cases of hypoalbuminemia it did not achieve five percent of this population. In addition to having a long half-life (20 days), albumin is an acute-phase protein that, during periods of inflammation (such as in obesity), may show increased serum levels, masking a possible presence of protein malnutrition. Thus, for bariatric surgery patients, a serum albumin level should not be the only parameter to consider when determining presence of protein deficiency, and should be analyzed with caution and in conjunction with other factors.13 However, serum albumin appears to be a more sensitive parameter in patients who have undergone malabsorptive procedures. Studies have shown a hypoalbuminemia prevalence rate of 13 percent rate among patients with super obesity two years after undergoing RYGB with a biliopancreatic loop of 150cm or greater. Prevalence increased to 18 percent in patients who underwent BPD, and in patients who had BPD/DS surgeries, the prevalence of severe protein calorie malnutrition is even higher at 20 to 30 percent.8
In addition to having a shorter half-life, prealbumin serum level (<0.2g/L) can be used to flag bariatric surgery patients prior to surgery who are at greater risk of becoming protein malnourished postoperatively.13 A recent observational study showed a drastic decrease in protein values among RYGB patients three months after surgery—37 percent and 57 percent of patients who underwent RYGB and SG, respectively, had mild protein depletion (prealbumin less than 0.2g/L), reaching postoperative stabilization at the end of six and 12 months, but remaining at low levels. In this same study, the prevalence of hypoalbuminemia was lower, seen in 21 percent and 16 percent of patients following RYGB and SG, respectively.6 In this sense, considering serum albumin level as the only diagnostic parameter for protein deficiency could prevent a large number of bariatric surgery patients from being properly diagnosed and treated for protein malnutrition.
Nutritional recommendation of protein intake after bariatric surgery. The American Association of Clinical Endocrinologists (AACE)/The Obesity Society (TOS)/American Society for Metabolic and Bariatric Surgery (ASMBS) Clinical Practice Guidelines for the Perioperative Nutritional, Metabolic, and Nonsurgical Support of the Bariatric Surgery Patient24 advocate a protein consumption of at least 60g/day or up to 1.5g/kg of ideal weight/day for weight loss with maintenance of muscle mass and prevention of protein malnutrition in patients who have had bariatric surgery.21,22 This recommendation is based mainly on the understanding that in low- and very-low-calorie, protein-rich diets, there is a greater preservation of lean mass in relation to isocaloric and normoproteic diets.22 Moize et al13 observed through multilinear regression analysis that protein intake was the only predictor of loss of muscle mass at the end of the first year in both RYGB and SG post-surgery patients. In addition, a protein intake greater than 60g/day, or 1.1g/kg body weight, was associated with a preservation of lean mass around five percent higher in both procedure study groups at four and 12 months postoperatively. Positive associations between total protein intake (g) and lean mass in more dysabsorptive procedures, such as BPD, have also been demonstrated.25
Indeed, little is known about the associations between changes in total energy intake and macronutrients (carbohydrates, fats, and proteins) and long-term weight changes in patients undergoing bariatric surgery. Most of the analyzed studies had low numbers of participants and did not measure long-term outcomes.26,27 To improve this scenario, data on dietary intake and weight change was recently published in the Swedish Obese Subjects (SOS) study, which followed more than 2,000 patients who underwent RYGB or SG for more than 10 years. The study showed that, when consuming the same amount of energy, patients at least six months postsurgery who favored protein consumption over consumption of carbohydrates or fat presented a weight loss approximately three percent higher after 10 years postoperative than those who favored the consumption of fats or carbohydrates over protein.28
Protein supplementation. Due to the restrictive characteristics of bariatric surgery, caloric consumption might decrease by as much as 60 percent during the first three months post-surgery compared to the preoperative phase, resulting in an average of about 800 calories consumed daily,6,20 which can make the recommended protein intake on a low-calorie diet challenging. The recent SOS observational study revealed interesting average caloric intake data of about 1600kcal at the end of a six-month postoperative period. However, even presenting a mean caloric intake that was apparently higher than previous observational studies (Table 1), protein consumption was still close to the lower limit of the current recommendations, around 70g/day.28 In Table 1, data from studies related to protein consumption at three, six, and 12 months post RYGB and SG are listed.
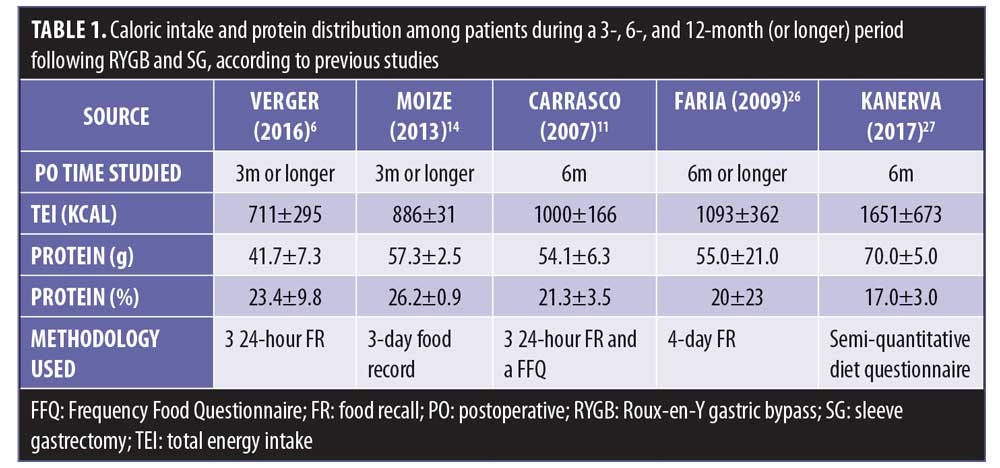
Moize et al13 reported that about 40 percent of patients who had RYGB or SG surgeries did not achieve the recommended minimum intake of 60g/day of protein during the first six months following their operation. More recent studies have reported even fewer patients achieving their recommended dietary protein intake,6,20 showing inadequate daily protein intake (less than 60g/day) in approximately 60 and 85 percent of patients post-RYGB or SG, respectively, at three and 12 months after surgery.29 A systematic review31 analyzed 729 patients after RYGB or SG and also found that most patients did not achieve 60g of protein intake daily.
In surgeries such as BPD, where the remnant gastric pouch is slightly larger (200–400mL), the prevalence of patients not meeting protein intake recommendation appears to be lower, at about 20 percent.25 It is important to note that food intake assessment instruments are subject to measurement biases and might influence the levels of evidence in the studies.
As a means to ease the difficulty in achieving adequate protein intake, protein supplements after bariatric surgery are often recommended to patients. Although patient adherence to taking the supplements is challenging and decreases even more with time,12,13,30 (e.g., only 30 percent of patients at the end of 12 months following RYGB or SG adhered to taking supplements), these seem to be effective instruments to help achieve the daily protein goal.12,20 A randomized clinical trial of women with post-RYGB weight regain demonstrated that the use of 15g/day of whey protein assisted in the management of the disease, leading to a greater fat loss with preservation of lean mass compared to the group that did not use the supplementation. Another recent randomized, controlled trial demonstrated that the group that took 30g of protein supplement per day lost more fat mass relative to total weight loss when compared to the placebo group at the end of the first year of bariatric surgery.31 Nevertheless, the use of protein supplements as support for weight loss after bariatric surgery requires additional research before strong conclusions can be made.
Protein uptake after RYGB. Interestingly, protein digestion and absorption appear not to be impaired after RYGB. A recent study using oral administration of intact casein as a protein source demonstrated an increase in aminoacidemia for four hours postprandial similar to preoperative levels. This elevation presented a higher and faster peak, but was also more transient in post-surgery patients, dropping to lower values than those at baseline about three hours after ingestion. The study suggests that gastric acid secretion and pepsin might not be essential to protein digestion, and secretions from the exocrine pancreas that are sent to the common limbus might be sufficient to effect proper digestion of the protein.20
It appears then that the pattern of protein turnover is altered after RYGB, since in this population, casein was rapidly absorbed, even though it was considered a slow absorption protein. Thus, the concept of slow and rapid absorption protein does not seem to apply to RYGB. It is known that the anabolic effect of protein intake depends on the digestion rate of this protein and the postprandial aminoacidemia profile, since the protein turnover is different in slow proteins (intact casein) and rapid absorption (whey protein). In this sense, it is possible that transient postprandial hyper aminoacidemia could potentially limit the phases of muscle anabolism over the course of 24 hours and aggravate the loss of lean mass during weight loss after RYGB. Therefore, it is always important to reinforce the need for small and frequent meals containing protein sources in the bariatric patient’s eating plan.32,33
Recommendations
- The diagnosis of protein deficiency should be done using biochemical parameters (albumin levels<3.5mg/d and prealbumin<0.2g/L), anthropometric, and clinical nutritional signs.
- Protein intake after bariatric surgery should be at least 60g/day. “And for a better approach, consumption levels greater than 1.1g/kg of ideal weight, namely from 1.5g to 2.1g/kg of ideal weight, could be considered.28
- The use of protein supplements might help to achieve these recommendations. It is recommended that they be used whenever the patient cannot obtain necessary nutrients from food sources, especially in the first six months postoperatively, when the loss of muscle mass is more intense.
- The consumption of food sources of protein must be present at each meal at three-hour intervals on average, together with a balanced diet and adequate caloric intake. This routine should be followed to facilitate the achievement of the daily protein intake goal, given the existence of the reduced size of the stomach, and also due to the possible increase in postprandial transience of amino acids levels demonstrated after RYGB. Adequate protein intake among patients who undergo bariatric surgery is important to ensure sufficient amino acid levels throughout the day, especially in the first postoperative year, when the energy balance will be negative and the risk of muscle mass loss is increased.
- Since the use of protein supplements is challenging, a wide variety of tastes and different products could help with adherence. Nutritional education should be used in motivating and teaching patients how to consume higher amounts of protein from their diets. Patients should learn how to calculate the amount of protein they are consuming (i.e., one egg has 5g of protein, a yogurt has 10g, and two slices of cheese have 10g). We have a wide variety of types of protein supplements available for the bariatric population: whey protein (isolated, concentrated, and blended), casein, albumin, beef protein, and vegan proteins. They present levels of more than 80 percent of protein. They should be used according to the patient’s tolerance and necessity. Different flavors should be used to increase adherence. The advantage of using protein supplementation is to increase protein intake without increasing fats and carbohydrates, besides making it easier to use since it can be taken in a liquid form.
Conclusion
Severe protein malnutrition (hypoalbuminemia) is rare, and parameters, such as prealbumin, intense weight loss, body composition, and clinical signs should be considered to detect an increased protein requirement. Protein digestion and absorption do not seem to suffer considerable losses after one of the most commonly performed bariatric surgical procedures, the RYGB, with low intake being the major risk factor for protein deficiency.
The ideal protein intake after bariatric surgery is difficult to achieve and appears to be inversely proportional to loss of muscle mass, but it does help in maintaining long-term weight loss, which can be aided through the use of protein supplements. Despite the existence of evidence from observational studies in favor of protein-rich diets after bariatric surgery, convincing evidence on the effect of protein ingestion on weight loss or other beneficial effects after RYGB, especially in the long term, is scarce, with only a small number of high-quality studies being published. Despite this, considering the increasing number of patients operated every year worldwide, the study of the influence of protein and its amino acid composition represent an important domain of knowledge development. Long-term, controlled intervention studies with adequate methodology (i.e., nitrogen balance) are necessary to establish a more reliable protein recommendation for this population.
In the nutritional follow-up, consideration should be given to teaching patients strategies to increase protein intake daily.
Acknowledgments
Silvia Leite Faria completed conception and design of the study, revision of the manuscript and approval of the final version. Mariane de Almeida Cardeal completed design of the study, and drafting and revision of the manuscript. Orlando Pereira Faria completed revision of the manuscript and approval of the final version.
References
- Welbourn R, Hollyman M, Kinsman R, et al. Bariatric surgery worldwide: baseline demographic description and one-year outcomes from the fourth IFSO Global Registry Report 2018. Obes Surg. 2019;29:782–795.
- Mingrone G, Bornstein S, Le Roux CW. Optimisation of follow-up after metabolic surgery. Lancet Diabetes Endocrinol. 2018;6(6):487–499.
- Mahawar KK, Sharples AJ. Contribution of malabsorption to weight loss after Roux-en-Y gastric bypass: a systematic review. Obes Surg. 2017;27(8):2194–2206.
- Chaston TB, Dixon JB, O’Brien PE. Changes in fat-free mass during significant weight loss: a systematic review. Int J Obes. 2007;31(5):
743–750. - Barazzoni R, Bischoff S, Boirie Y, et al. Sarcopenic obesity: time to meet the challenge. Obes Facts. 2018:294–305.
- Aron-Wisnewsky J, Verger EO, Bounaix C, et al. Nutritional and protein deficiencies in the short term following both gastric bypass and gastric banding. PLoS One. 2016;11(2).
- Sjöström L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351(26):2683–2693.
- Fox SR. The use of the biliopancreatic diversion as a treatment for failed gastric partitioning in the morbidly obese. Obes Surg Incl Laparosc Allied Care. 1991;1(1):89–93.
- Gomes DL, Moehlecke M, Bassan F, et al. Whey protein supplementation enhances body fat and weight loss in women long after bariatric surgery : a randomized controlled trial. Obes Surg. 2017;27(2):424–431.
- Faria SL, Faria OP, Buffington C, et al. Energy expenditure before and after Roux-en-Y gastric bypass. Obes Surg. 2012:1450–1455.
- Carrasco S, Papapietro K, Csendes A, et al. Changes in resting energy expenditure and body composition after weight loss following Roux-en-Y gastric bypass. Obes Surg. 2007;17:608–616.
- Ito MK, Siqueira V, Gonçalves S, et al. Effect of protein intake on the protein status and lean mass of post-bariatric surgery patients: a systematic review. Obes Surg. 2017; 27(2):
502–512. - Moize V, Geliebter A, Gluck ME, et al. Obese patients have inadequate protein intake related to protein intolerance up to 1 year following Roux-en-Y gastric bypass. Obes Surg. 2003;13(1)23–28.
- Moizé V, Andreu A, Rodríguez L, et al. Protein intake and lean tissue mass retention following bariatric surgery. Obes Surg. 2013;32:550–555.
- Raftopoulos I, Bernstein B, O’Hara K, et al. Protein intake compliance of morbidly obese patients undergoing bariatric surgery and its effect on weight loss and biochemical parameters. Surg Obes Relat Dis. 2011;7(6):733–742.
- Nicoletti CF, De Oliveira BAP, Barbin R, et al. Red meat intolerance in patients submitted to gastric bypass: a 4-year follow-up study. Surg Obes Relat Dis. 2015;11(4):842–846.
- Davidson LE, Yu W, Goodpaster BH, et al. Fat-free mass and skeletal muscle mass five years after bariatric surgery. Obesity. 2018;26(7):1130–1136.
- Cole AJ, Kuchnia AJ, Beckman LM, et al. Long-term body composition changes in women following Roux-en-Y gastric bypass surgery. J Parenter Enter Nutr. 2017;41(4):583–591.
- Ritz P, Vaurs C, Barigou M, Hanaire H. Hypoglycaemia after gastric bypass: mechanisms and treatment. Diabetes Obes Metab. 2016;18(3):217–223.
- Bojsen-Møller KN, Jacobsen SH, Dirksen C, et al. Accelerated protein digestion and amino acid absorption after Roux-en-Y gastric bypass. Am J Clin Nutr. 2015;102(3):600–607.
- Steenackers N, Gesquiere I, Matthys C. The relevance of dietary protein after bariatric surgery : what do we know? Curr Opin Clin Nutr Metab Care. 2018;21(1):58–63.
- Via MA, Mechanick JI. Nutritional and micronutrient care of bariatric surgery patients: current evidence update. Curr Obes Rep. 2017;6(3):286–296.
- Levinson R, Silverman JB, Catella JG, et al. Pharmacotherapy prevention and management of nutritional deficiencies post Roux-en-Y gastric bypass. Obes Surg. 2013;23(7):
992–1000. - Sugerman H, Anderson W, Collazo-Clavell M, et al. American association of clinical endocrinologists, the obesity society, and american society for metabolic & bariatric surgery medical guidelines for clinical practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric. Endocr Pract. 2013;14(Suppl 1):1–83.
- Obeid NR, Malick W, Concors SJ, Fielding GA, et al. Long-term outcomes after Roux-en-Y gastric bypass: 10- to 13-year data. Surg Obes Relat Dis. 2016;12(1):11–20.
- Faria SL, De Oliveira Kelly E, et al. Nutritional management of weight regain after bariatric surgery. Obes Surg. 2010;20(2):135–139.
- Kanerva N, Larsson I, Peltonen M, et al. Changes in total energy intake and macronutrient composition after bariatric surgery predict long-term weight outcome: findings from the Swedish obese subjects (SOS) study. Am J Clin Nutr. 2017;106(1):136–145.
- Sherf Dagan S, Keidar A, Raziel A, et al. Do bariatric patients follow dietary and lifestyle recommendations during the first postoperative year? Obes Surg. 2017;27(9):2258–2271.
- Schollenberger AE, Karschin J, Meile T, et al. Impact of protein supplementation after bariatric surgery: A randomized controlled double-blind pilot study. Nutrition. 2016;32(2):186–192.
- Hood MM, Kelly MC, Feig EH, et al. Measurement of adherence in bariatric surgery: a systematic review. Surg Obes Relat Dis. 2018;14(8):1192–1201.
- Cardeal M de A, Faria SL, Faria OP, et al. Diet-induced thermogenesis in postoperatve Roux-en-Y gastric bypass patients with weight regain. Surg Obes Relat Dis. 2016;12(5):
1098–1107. - Dagan SS, Goldenshluger A, Globus I, et al. Nutritional recommendations for adult bariatric: clinical practise. Adv Nutr. 2017;8(12):382–394.
- Pérez-Martínez P, Mikhailidis DP, Athyros VG, et al. Lifestyle recommendations for the prevention and management of metabolic syndrome: an international panel recommendation. Nutr Rev. 2017;75(5):
307–326.
Category: Past Articles, Review



