Weight Loss Medications for Patients: A Review
 This column is written by medical students and is dedicated to reviewing the science behind obesity and bariatric surgery.
This column is written by medical students and is dedicated to reviewing the science behind obesity and bariatric surgery.
by Manoj Kanagaraj, BS, BA
Medical Student, Harvard Medical School, Boston, Massachusetts
Column Editor: Daniel B. Jones, MD, MS, FASMBS
Professor of Surgery, Harvard Medical School, Vice Chair, Beth Israel Deaconess Medical Center Boston, Massachusetts
Funding: No funding was provided for this article.
Disclosures: The authors have no conflicts of interest relevant to the content of this article.
Abstract: Obesity is among the most common causes of morbidity in the United States. Few patients and providers are aware of effective treatment options for obesity besides bariatric surgery and lifestyle interventions. This review will focus on the use of medications for weight loss in patients with obesity. It will address the indications, options, costs, benefits, and effectiveness of United States Food and Drug Administration (FDA)-approved weight loss medications currently on the market.
Keywords: Weight loss, drugs, pharmacotherapy, price, medication
Bariatric Times. 2018;15(4):8–10.
Introduction
Obesity is one of the most common causes of disability today. In the United States alone, nearly two-thirds of Americans have overweight or obesity.1 Obesity is also reaching epidemic proportions worldwide as well.2 Weight loss can help patients with obesity avoid suffering from such medical conditions as heart disease, diabetes, and cancer.1 It is critical that effective treatment options exist to help patients tackle this problem.
Obesity, however, is difficult to treat and often requires multiple therapeutic modalities. The first measures usually involve intensive lifestyle interventions, including strict diet, exercise, and cognitive changes to promote healthy eating and increased activity. Later measures can include a combination of lifestyle intervention and bariatric surgery, which is highly effective for both weight loss and control of associated comorbidities.3 Few other options exist for patients seeking a bridge between behavioral changes and invasive surgery. Chief among them are pharmacotherapies. Currently, there are six United States Food and Drug Administration (FDA)-approved medications for weight loss.4 These medicines are primarily intended either as adjuncts to diet or exercise.5
Weight loss medicines have a notably checkered past. In the 1930s, 2,4-dinitrophenol (DNP) gained considerable popularity for its weight-reducing effects.6 It works by interfering with the process that creates adenosine triphosphate (ATP). Specifically, DNP uncouples oxidative phosphorylation by increasing the basal leakage of protons across the mitochondrial membrane. This weakens the proton gradient that is necessary to fuel ATP production. The potential energy from the proton gradient is instead lost as heat. Consequently, more calories must be consumed per unit of energy produced. The medicine’s weight-loss effect thus came coupled with the side effect of hyperthermia. Many patients presented with hyperthermia, cataracts, tachycardia, diaphoresis, tachypnea, and eventual cardiac arrest.7 By 1938, the FDA recommended against human usage of DNP.
Multiple weight loss compounds have since been studied, some of which have undergone clinical trials and been approved by the FDA for the treatment of obesity. Before prescribing any of these medications to your patients, it is important to consider the risks versus benefits of each in order to determine the safest and most effective medicine for each patient. This review addresses this critical concern in the management of the bariatric patient.
Indications
Pharmacologic therapy is indicated for any patient diagnosed with obesity (body mass index [BMI] ≥30).8 Some patients who are moderately overweight (BMI ≥27) but do not have obesity can use certain weight-loss drugs if they have at least one co-existing condition associated with obesity. These conditions include diabetes, hypertension, hyperlipidemia, and sleep apnea.9
If the appropriate criteria are met, patients with obesity should be started on these medications when they fail to reach adequate weight loss through lifestyle intervention alone.4 Importantly, lifestyle intervention must continue even while patients use prescription weight loss drugs. These medicines achieve best results when used as adjuncts to a weight-loss program that is already incorporating increased exercise, behavioral therapy, and calorie-restricting diets. These medications should be discontinued if less than five percent of weight is lost in a 12-week trial.10
Options
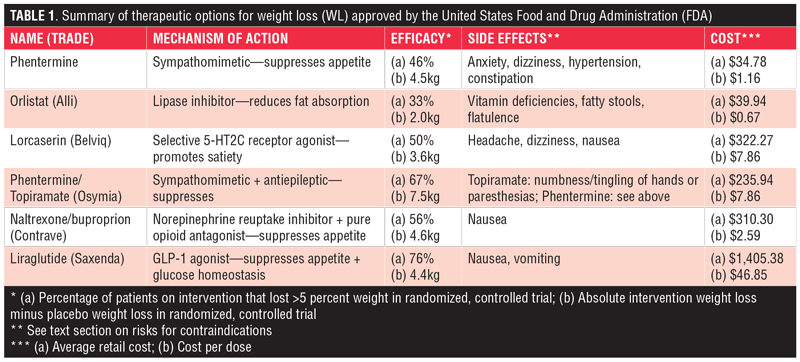
Phentermine. First introduced in 1959, phentermine is the most commonly prescribed weight loss medication.4 It works to reduce appetite through its sympathomimetic properties. This suppression of appetite helps decrease food consumption and weight gain. A clinical trial in 2013 demonstrated that phentermine monotherapy led to at least five percent weight loss in 43 percent of patients on a low dose formulation and 46 percent on a higher dose formulation.11 The side effects of the drug are similar to those of other stimulants and include dizziness, anxiety, insomnia, tachycardia, hypertension, diarrhea, or constipation. Notably, phentermine had been historically used in tandem with an anti-obesity medication called fenfluramine that was withdrawn from the market for increasing risk of cardiac valvulopathy in patients.12 Most of this risk was attributed to the fenfluramine component of the drug combination, leaving phentermine available on the market. Phentermine is approved by the FDA in a lower-dose, 8mg formulation called Lomaira. It is implied that this formulation leads to similar outcomes with reduced side effects based on historical trials, which is why the FDA approved this medicine. Lomaira itself, however, has not been specifically tested in clinical trials, making these claims debatable.4 Phentermine is one of the more affordable weight-loss medications on the market, with an average retail price of $34.78 for 30 tablets (cost per dose: $1.16). With coupon, this medicine is available for $10.26.13
Orlistat (Alli). Orlistat (marketed as Alli or Xenical) is a lipase inhibitor approved by the FDA first in 1999 as a prescription drug for obesity management and again in 2007 as an over-the-counter medication for weight loss. Orlistat achieves its effect by interrupting the absorption of fat in the gastrointestinal system. A double-blind, randomized, controlled trial in 1999 demonstrated that a third of patients who received the drug lost greater than five percent of their body weight and saw a significant drop in their serum lipid levels.14 The interference of fat absorption led to some predictable side effects, including fatty stools, flatulence, oily spotting, and fat-soluble vitamin deficiencies, among others.15 Orlistat is the most affordable weight-loss drug with an average retail price of $39.94 for 60 pills (cost per dose: $0.67).16
Lorcaserin (Belviq). Approved in June 2012, lorcaserin (trade name Belviq) works by centrally suppressing appetite. Specifically, lorcaserin selectively activates the 5-HT2C receptors in the pro-opiomelanocortin neurons of the hypothalamus to promote satiety. In a 2010 trial, nearly 50 percent of lorcaserin-treated patients lost five percent or more of their body weight compared to only 20 percent of placebo-treated patients.17 Adverse effects of the drug were minimal, with most patients citing headache, dizziness, and nausea as their primary complaints. Few patients experienced the adverse cardiovascular events that less selective drugs of the serotonin class like fenfluramine caused.4 Belviq has an average retail price of $322.27 for 60 tablets (cost per dose: $5.37). Patients can use a free coupon to receive 13 percent off the medication, bringing the price down to $279.39.18
Phentermine/topiramate (Qsymia). In July of 2012, phentermine was approved for weight loss yet again. This time, it achieved an improved therapeutic effect in combination with topiramate, an antiepileptic drug known to induce anorexia. While the exact mechanism of action is not clearly understood, the drug combination led to a significantly higher proportion of patients achieving greater than five percent weight loss compared to placebo (67% vs 17%, respectively).19 Drug-treated patients also saw significant reductions in hypertension and hyperlipidemia. Major adverse effects included insomnia, dry mouth, and constipation.4 Just as phentermine can be independently taken for weight loss, so too can topiramate. Topiramate alone can achieve the appetite suppression that Qsymia produces. This strategy should be employed when patients cannot use phentermine due to side effects or contraindications.11 Importantly, though, the efficacy of this drug combination reported above is specific to the Qsymia combined formulation. The effect is not as significant when phentermine and topiramate are separately prescribed but simultaneously taken. The average retail price of Qsymia is $235.94 (cost per dose: $7.86), although discounts bring the price down to $193.25. Some patients may be prescribed the components of the combination individually for a cheaper overall price, despite decreased weight-loss effectiveness.20
Naltrexone/buproprion (Contrave). The naltrexone/buproprion combination drug was approved in September 2014 under the trade name Contrave. It achieved its therapeutic effect by targeting the same pro-opiomelanocortin neurons that lorcaserin acted on. Uniquely, though, the naltrexone component of Contrave antagonized opioid receptors to augment buproprion’s activation of those neurons. The effect led to significantly reduced food craving and appetite suppression in patients. Approximately 56 percent of patients treated with Contrave lost greater than five percent of their weight compared to only 18 percent on placebo.21 The most common side effect patients reported was nausea, with minimal other adverse effects. The average retail price is $310.30 for 120 tablets (cost per dose: $2.59), but free rebates can bring the price down to $234.69.22
Liraglutide (Saxenda). Liraglutide, a GLP-1 agonist originally approved for Type 2 diabetes management, is an injectable therapy that both promotes satiety and affects glucose homeostasis. Liraglutide is produced by Novo Nordisk (Bagsværd, Denmark) in two formulations: Victoza and Saxenda. Victoza is used for glucose homeostasis in diabetes management. Saxenda is specifically indicated for weight loss. Saxenda’s 3.0mg dose versus Victoza’s maximum 1.8mg dose make Saxenda more effective at promoting weight loss. In 2014, the results of a double-blind, placebo-controlled trial demonstrated that 76 percent of patients on Saxenda lost greater than five percent of their weight compared to only 30 percent on placebo.23 Moreover, Saxenda reduced both hypertension and hyperglycemia in treated patients. Nausea and vomiting were the most common adverse effects, with the therapy being otherwise well tolerated. Saxenda for weight loss is by far the most expensive prescription weight loss medication on the market. Its average retail price is $1,405.38 for 30 injections (cost per dose: $46.85), but coupons can bring the cost down to $1,233.57 and additional rebates are also available.24
Risks
In addition to the side effects of prescription weight loss drugs mentioned above, ranging from nausea to stimulant-like effects of phentermine to even the fatty stools and vitamin deficiencies caused by orlistat, there are other considerable risks of these medicines that should be kept in mind. Accordingly, patients with particular conditions that could be worsened by these side effects are contraindicated from taking those medications. Patients with cardiovascular disease, hyperthyroidism, history of drug abuse, or who are currently on monoamine oxidase inhibitors (MAOIs) or tricyclic antidepressants are recommended against using phentermine, phentermine/topiramate, or naltrexone/bupropion.4 Those with cholestasis or malabsorption problems should not use orlistat. Pregnant patients should avoid using all weight-loss drugs.10 Liraglutide is contraindicated in individuals with a family or personal history of thyroid malignancy or multiple endocrine neoplasia Type 2 (MEN2) because of its association with medullary thyroid cancer.4 Most of the medicines with the worst reported adverse effects have been withdrawn from the market, including sibutramine for increasing risk of cardiovascular disease, rimonabant for increasing risk of depression, and fenfluramine for increasing risk of cardiac valvulopathies and primary pulmonary hypertension.25,26
Conclusion
Weight-loss therapeutics are important tools in obesity management. They offer auxiliary support for patients struggling to achieve their weight loss goals through diet, exercise, and behavior changes alone. Most of them help patients lose 3 to 7 percent of their excess body weight in the long term. Importantly, though, these medications are only successful when coupled with intensive lifestyle intervention. Additionally, their risks are not insignificant. Patients with certain comorbidities must be careful to avoid anti-obesity medicines that will have adverse effects on them. Given the history of hazardous drugs for weight loss, physicians are likely to be more reluctant prescribing these therapies for their patients. And although most of these medicines are reasonably priced, the more recently approved ones are less affordable. While rebates and discounts can make these drugs more affordable, patients without adequate insurance coverage might struggle to purchase them for an extended period of time.
Of the options for weight loss available to patients with obesity, lifestyle interventions remain the most safe and accessible and bariatric surgery remains the most efficacious. The reviewed medicines, while helpful as adjuncts, are not effective enough to be considered first-line. These drugs are, however, helpful for patients who do not meet the more stringent BMI criteria of 40 or 35 with comorbidities for bariatric surgery. They can also be considered for patients who need assistance with maintaining weight loss after receiving bariatric surgery, although no specific trials have been conducted to measure the efficacy of such a regimen.
With the varied risks and benefits of each of the six FDA-approved prescription and over-the-counter weight-loss medicines available today, it is important for providers to individually tailor medication regimens to their patients’ unique needs. Patients with obesity remain in need of novel weight-loss therapeutics that are more effective and maintain low risk profiles.
Several new drugs not yet approved by the FDA for weight loss are being used effectively off label, including semaglutide (Ozempic).28 Soon, these medicines might become officially indicated for anti-obesity therapy by the FDA. As we continue to gain insights into pathophysiology of obesity, perhaps we will encounter new therapeutic targets for which drugs can be made. These efforts are essential for offering hope to patients around the world looking for assistance with managing obesity.
References
- Moyer VA, LeFevre ML, Siu AL, et al. Screening for and management of obesity in adults: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2012;157:373–378.
- Tsigos C, Hainer V, Basdevant A, et al. Management of obesity in adults: European clinical practice guidelines. Obesity Facts. 2008;1:106–16
- Chang SH, Stoll CR, Song J, et al. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA Surg. 2014;149:275–87.
- Srivastava, Apovian CM. Current pharmacotherapy for obesity. Nat Rev Endocrinol. 2018;14:12–24.
- Stanford F, Alfaris N, Gomez G, et al. The utility of weight loss medications after bariatric surgery for weight regain or inadequate weight loss: a multi-center study. Surg Obes Relat Dis. 2017;13:491–500.
- Tainter ML, Cutting WC, Stockton AB. Use of dinitrophenol in nutritional disorders: a critical survey of clinical results. Am J Public Health Nations Health. 1934;24:1045–53.
- Grundlingh J, Dargan PI, El-Zanfaly M, Wood DM. 2,4-dinitrophenol (DNP): a weight loss agent with significant acute toxicity and risk of death. J Med Toxic. 2011;7:205.
- Eckel R. Nonsurgical management of obesity in adults. N Engl J Med. 2008;358:1941–50.
- Yanovski S, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA. 2014;311:74–86.
- Bersoux S, Byun TH, Chaliki SS, Poole KG. Pharmacotherapy for obesity: what you need to know. Cleve Clin J Med. 2017;84:951–8.
- Aronne LJ, Wadden TA, Peterson C, et al. Evaluation of phentermine and topiramate versus phentermine/topiramate extended-release in obese adults. Obesity (Silver Spring). 2013;21:2163–71
- Connolly HM, Crary JL, McGoon MD, et al. Valvular heart disease associated with fenfluramine–phentermine. N Engl J Med. 1997;337:581–8.
- Prices for Phentermine. GoodRx. 2018; https://www.goodrx.com/phentermine.
- Finer N, James WP, Kopelman PG, et al. One-year treatment of obesity: a randomized, double-blind, placebo-controlled, multicentre study of orlistat, a gastrointestinal lipase inhibitor. Int J Obes Relat Metab Disord. 2000;24:306–13.
- Davidson MH, Hauptman J, DiGirolamo M, et al. Weight control and risk factor reduction in obese subjects treated for 2 y with orlistat: a randomized controlled trial. JAMA. 1999;281:235–42.
- Prices for Alli. GoodRx. 2018; https://www.goodrx.com/alli?drug-name=alli.
- Smith SR, Weissman NJ, Anderson CM, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363:245–56.
- Prices for Belviq. GoodRx. 2018; https://www.goodrx.com/belviq?drug-name=belviq.
- Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20:330–42.
- Prices for Qsymia. GoodRx. 2018; https://www.goodrx.com/qsymia?drug-name=qsymia.
- Apovian CM, Aronne L, Rubino D, et al. A randomized, Phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity (Silver Spring). 2013;21:935-43.
- Prices for Contrave. GoodRx. 2018; https://www.goodrx.com/contrave?drug-name=contrave.
- Astrup A, Rössner S, Van Gaal L, et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374:1606–16.
- Prices for Saxenda. GoodRx. 2018; https://www.goodrx.com/saxenda?drug-name=saxenda.
- Ioannides-Demos LL, Proietto J, Tonkin AM, et al. Safety of drug therapies used for weight loss and treatment of obesity. Drug Safety. 2006;29:277-302.
- Beyer CE, Dwyer JM, Piesla MJ, et al. Depression-like phenotype following chronic CB1 receptor antagonism. Neurobio of Dis. 2010;39:148–55.
- Nguyen NT, Varela JE. Bariatric surgery for obesity and metabolic disorders: state of the art. Nat Rev Gastroenterol Hepatol. 2017;14:160–9.
- Blundell J, Finlayson G, Axelsen M, et al. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes Metab. 2017;19:1242-51.
- Cefalu WT, Stenlöf K, Leiter LA, et al. Effects of canagliflozin on body weight and relationship to HbA1c and blood pressure changes in patients with type 2 diabetes. Diabetologia. 2015;58:1183–7.
Category: Medical Student Notebook, Past Articles



